| Posted: Sep 20, 2007 | |
Rechargeable molecular cluster batteries |
|
| (Nanowerk Spotlight) Lithium-ion (Li-ion) batteries are a type of rechargeable battery commonly used in consumer electronics. They are currently one of the most popular types of battery for portable electronics, with one of the best capacity-to-weight ratios, no memory effect, and a slow loss of charge when not in use. Lithium is useful in batteries because of its lightness (it is the lightest metal) and because of the high voltage of the redox reaction between Li and Li+. In lithium ion batteries, a layered compound - lithium copper oxide or or lithium nickel oxide - is utilized as a cathode. Although this material can provide high capacity, its charging/discharging rates are slow because these processes include the absorption/desorption of lithium in the cathode. Recently, organic radical batteries have been developed as a new type of rechargeable battery, in which organic radical polymers are utilized as a cathode active material. They achieved a very fast chargeable/dischargeable rate, though their capacities are lower than those of the lithium ion batteries. A lot of research has gone into fabricating lithium batteries that achieve both high capacity and fast charging/discharging. Researchers in Japan came up with a completely new idea - the molecular cluster battery - where the cathode active material is a well-known manganese molecular cluster that is stable and insoluble to most solvents and exhibits a multi-step redox reaction. Although the battery was rechargeable, in early experiments the fast charging–discharging was not yet achieved due to the chemical decomposition of the cluster. Nevertheless, this is a first step that opens up a new branch of research into high-performance rechargeable molecular cluster batteries. | |
| Molecular metal clusters are small aggregates of metal ions connected by (organic) ligands. Some of these clusters are high-spin species that have attracted much attention among scientists as potential single molecule magnets in the field of molecular magnetism. | |
| "To achieve both high capacity and fast charging/discharging batteries, we thought of adopting molecule-based compounds undergoing multi-step redox reactions as a cathode active material" Dr. Kunio Awaga explains to Nanowerk. "We wanted to replace the organic radical moieties in the organic radical batteries with transition metal cluster complexes which can accommodate many electrons. We looked at Mn12 – [Mn12O12(CH3COO)16(H2O)4] to be precise – which has been extensively studied as a single molecule magnet, and succeeded in demonstrating a new application of Mn12 in solid state electrochemistry." | |
 |
|
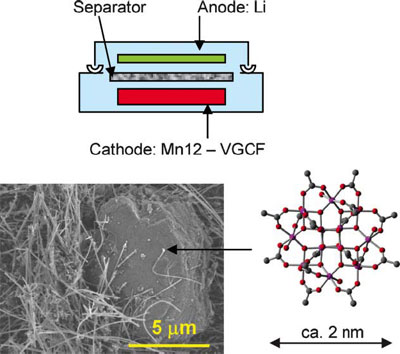
| SEM image of the cathode. The cartoon schematically shows the structure of the Mn12–Li molecular cluster battery. (Reprinted with permission from RSC Publishing) | |
| Awaga, a professor at the Research Center for Materials Science & Department of Chemistry at Nagoya University, together with colleagues from his Center as well as collaborators from the Nano Electronics Research Laboratories at NEC Corporation, and Nippon Kasei Chemicals, fabricated a battery combing a lithium anode and a cathode consisting of Mn12 and VGCF (vapor grown carbon fibers), and then carried out charging/discharging experiments. | |
| The Japanese researchers reported their findings in Chemical Communications ("Rechargeable molecular cluster batteries"). | |
| Experiments showed that the molecular cluster batteries have a capacity of ∼90 Ah/Kg, as well as 200-250 Ah/Kg being observed for the first discharging process. In comparison, typical lithium ion batteries have a capacity of approximately 180 Ah/kg. | |
| "We found a rechargeable battery performance with a moderate capacity, while the first discharging process exhibited an extremely high value" says Awaga. "This is the first study to demonstrate that molecular cluster species have the ability to act as cathode active materials." | |
| At the moment, the cathode is just a mixture of microcrystals of Mn12 and VGCF, and the connections between them are poor. Awaga says that, probably due to this fact, there was a serious decomposition of Mn12 under rapid charging/discharging experiments. To achieve rapid charging/discharging, the researchers need to improve the connections. | |
| Apart from Mn12, a lot of the transition metal cluster complexes have been recently synthesized mainly for the purpose of studying single molecule magnets. Awaga is sure that some of them are promising as cathode active materials. | |
| "In the near future" he says, "the importance and usefulness of molecular clusters as cathode active materials will be widely recognized among synthetic inorganic chemists, and extensive material research will be carried out. This will develop high-performance, rechargeable molecular cluster batteries to the level of practical application." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
