| Posted: Nov 20, 2012 | |
Truly blond - hair as a nanoreactor to synthesize gold nanoparticles |
|
| (Nanowerk Spotlight) In nature, numerous inorganic materials are synthesized by living organisms. These bioinorganic materials can be extremely complex both in structure and function, and also exhibit exquisite hierarchical ordering from the nanometer to macroscopic length scales which has not even remotely been achieved in laboratory-based syntheses. Inorganic materials in the form of hard tissues are an integral part of most multicellular biological systems. | |
| The possibility of using such microorganisms and plants in the deliberate synthesis of nanomaterials is a recent phenomenon and scientists are now exploring the use of biological organisms and materials to literally grow nanomaterials (see our previous Nanowerk Spotlight: "Truly green nanotechnology – growing nanomaterials in plants"). | |
| In a novel approach, researchers have now synthesized nanoparticles in hair. The purpose was to try to describe some of the chemical reactions occurring inside the hair shaft, in the so-called amorphous matrix surrounding intermediate filaments made of keratin proteins. This matrix can be seen as a set of nanoreactors. | |
| The team of scientists from CNRS reported their findings in the November 2, 2012 online edition of Nano Letters ("Hair Fiber as a Nanoreactor in Controlled Synthesis of Fluorescent Gold Nanoparticles") | |
 |
|
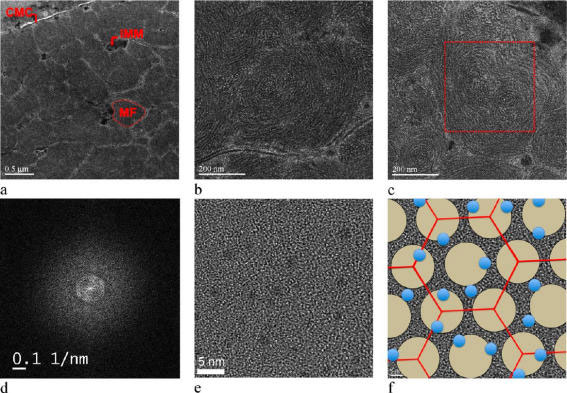
| In the cortex (a,b,c: HAADF) the macrofibril (MF) boundaries are decorated by gold that is also present in the MF bulk. (d) Fourier transform of the image identified by the red square in image (c), showing a ring at a position between 11 and 12 nm. (e) Observation in bright field at the atomic resolution of the gold nanoparticles in the cortex. (f) Model of the gold nanoparticles distribution in the cortex according to the hexagonal paracrystal model of the intermediate filaments (beige circles) arrangement and the location of the gold nanoparticles from e represented by blue circles. (Reprinted with permission from American Chemical Society) | |
| "Our study shows that the composition and the structure of keratins induce the synthesis of nanoparticles with an homogeneous size in the cuticle (2.5nm) and in the cortex (about 1.5 nm)," Dr. Philippe Walter, director of the Laboratoire d’archéologie moléculaire et structurale, LAMS, CNRS UMR 8220, tells Nanowerk. "This is due their environment, i.e. to the binding with sulfur from cystine amico-acids." | |
| Walter adds that the idea to use gold nanoparticles to dye wool was already published and patented by the group of James H. Johnston in New Zealand with differently sized nanoparticles. | |
| Previously, Walter and his team used these nanoreactors to form PbS quantum dots (we reported on this in our Spotlight: "Nanotechnology in cosmetics – 2000 years ago...? ") as well as HgS nanoparticles ("Characteristics of HgS nanoparticles formed in hair by a chemical reaction"). | |
| In this new work by the French team, the synthesis occurs in situ, inside the shaft. They were able to achieve a variation of color, from gold/blonde, to brown and dark brown. | |
| "We observe the self-organization of the nanoparticles along the fiber axis due to the structure of intermediate filaments of keratin molecules," explains Walter. "We started with the idea of trying with gold salts to use another way to check the chemical properties of keratins and of their supramolecular assemblies. Our results highlight the possible use of these chemical conditions to produce gold nanoparticles from gold salts in a one-pot nucleation, growth, morphogenesis, and passivation of extremely small gold nanoparticles." | |
| He notes that even multiple washings of treated hairs did not affect the color of the hair as the colored gold nanoparticles are buried and stabilized inside the hair by the keratin structure. | |
| This work has been part of a multidisciplinary project: it aims at understanding the cause of the degradation of keratin-based materials – hair and stratum corneum of mummies, feather, archaeological and ethnographic artifacts – and what role the supramolecular organization of hair plays at scales ranging from nanometers to micrometers. | |
| Because the gold nanoparticles formed inside the hair are fluorescent under UV light, researchers can now consider a number of applications. | |
| "For instance, we can look at the quenching of this fluorescence after an uptake of metallic cations by the hair shaft – for example mercury and arsenic – and develop new sensors for toxic elements in water," says Walter. "We can also extract the nanoparticles and use the hair as a template for the synthesis of nanoparticles with a narrow distribution of size." | |
| Going forward, the team would like to use the synthesis of the nanoparticles to check the preservation of the structure of archaeological hair. | |
| "One question we would like to answer is if the sizes of the nanoparticles are the same after several thousand of years of alteration," says Walter. "Here, the synthesis of nanoparticles can be considered as a way to characterize the chemical modifications in the fiber." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
