| Posted: Jan 10, 2013 | |
Nanotechnology and the environment - transformation of nanomaterials |
|
| (Nanowerk Spotlight) Assessing the environmental and human health implications of engineered nanomaterials requires an understanding of the potential exposure routes (read more: "Toxicology - from coal mines to nanotechnology"). These could vary during its lifecycle. During the manufacture of a product, free engineered nanoparticles and carbon nanotubes might be present in the air during production, or might be released into the environment with waste materials or during production accidents. Product use could lead to exposure to engineered nanoparticles through the skin (cosmetic products), ingestion (food ingredients or packaging) or injection (medical procedures). After regular use, recycling or degradation of products might release engineered nanoparticles into the environment and lead to high concentrations in water, air or soil, which in turn could lead to exposure through skin, inhalation or ingestion. | |
| To date, the predominant focus of the nanotechnology risk research endeavor has been defining the fate, transport, and toxic properties of pristine or “as manufactured” nanomaterials. However, the high surface to volume ratio and reactivity of nanoparticles makes them highly dynamic in environmental systems. The resulting transformations of the nanomaterials will affect their fate, transport, and toxic properties. | |
| A recent review published in Environmental Science & Technology ("Transformations of Nanomaterials in the Environment") summarizes what is known about chemical, physical, and biologically mediated transformations of nanomaterials in natural systems and their effects on the resulting nanomaterial behavior. | |
| The authors also discuss state-of-the-science knowledge and instrumentation gaps preventing scientists from quantifying and predicting these transformations in biological and environmental media. | |
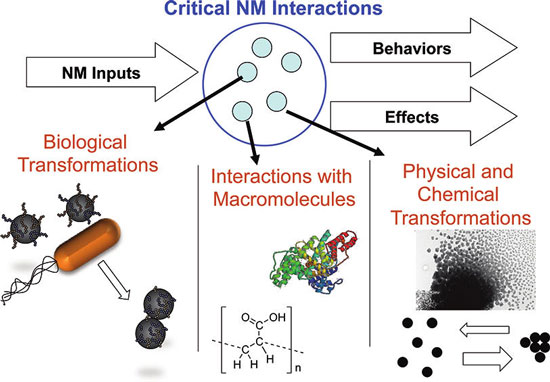 |
|
| Nanomaterial transformations are critical processes affecting nanomaterial interactions. Transformations include physical and chemical transformations, biologically mediated transformations, and interactions with macromolecules and biomacromolecules. (Reprinted with permission from American Chemical Society) | |
| Chemical transformations | |
| Reduction and oxidation are coupled processes in natural systems and involve the transfer of electrons to and from chemical moieties. A number of nanomaterials may be composed of or contain constituents that undergo reduction, oxidation, or both in aquatic and terrestrial environments. These include elemental metal nanomaterials such as silver and iron. | |
| Sunlight-catalyzed redox reactions (photooxidation and photoreduction) may prove to be very important transformation processes affecting nanomaterial coatings, oxidation state, generation of reactive oxygen species (ROS), and persistence. | |
| Dissolution and sulfidation are important processes affecting nanoparticle surface properties, toxicity, and persistence. | |
| Adsorption of macromolecules or organic and inorganic ligands on nanomaterial surfaces can significantly affect their surface chemistry and resulting behavior in biological and environmental systems. For example, adsorption of polymer coatings on nanoparticles generally decreases their attachment to silica surfaces, suggesting greater mobility in the environment and potentially less effective removal in drinking water treatment. | |
| Physical transformations | |
| Aggregation of nanoparticles reduces the surface area to volume effects on nanomaterial reactivity. This increase in aggregate size in turn affects their transport in porous media, sedimentation, reactivity, uptake by organisms, and toxicity. Over time, aggregation of nanoparticles into clusters is inevitable without engineered or incidental coatings to decrease aggregation. | |
| Aggregation can also decrease the “available” surface area of the materials, thereby decreasing reactivity. Aggregation can therefore decrease toxicity when the toxic response is a result of a surface area-mediated reaction such as ROS generation or dissolution. Aggregation may also serve to increase the persistence of the nanomaterial if aggregation decreases the rate of dissolution or degradation, albeit in a different location compared to the dispersed nanoparticles. | |
| Delineating the effects of aggregation on uptake and any subsequent toxicity will be challenging since it is a dynamic process, uptake will be highly dependent on both the species examined and its aqueous chemical environment and metabolic state, and because instruments for tracking nanomaterials in situ or in vivo are lacking. | |
| Biologically mediated transformations | |
| Biological transformations of nanomaterials are inevitable in living tissues (both intracellular and extracellular) and environmental media (e.g., soils). | |
| Redox reactions are fundamental to growth in all biological systems. The redox reactions between bacteria and naturally occurring, nanoscale iron oxide are well understood. | |
| Biologically mediated transformations of both the underlying nanomaterial core and the coatings are possible, and these transformations can affect the behavior of the nanomaterials including surface charge, aggregation state, and reactivity, which ultimately can affect transport, bioavailability, and toxicity. | |
| Biotransformation of polymer coatings used on many nanomaterials for biomedical applications is also feasible. Biological transformations of nanomaterials, especially carbon-based ones, and their organic coatings may ultimately act to attenuate their concentrations in the environment or to affect transport, but it remains to be seen if these processes occur at rates that are high enough to be important. | |
| Macromolecule nanomaterial interactions | |
| Perhaps the most critical biotransformation of nanomaterials is adsorption of biomacromolecules on their surfaces. | |
| There are an endless number of biomacromolecules in living cells (e.g., proteins) and in the environment (e.g., natural organic matter, polysaccharides). Adsorption of these macromolecules can occur in all environments. On uptake by biological organisms, nanomaterials may be transformed through their interaction with biomacromolecules which can coat and thereby transform their outer surfaces. | |
| Once discharged into the environment, uncoated or coated nanomaterials will be subjected to alterations through interactions with naturally occurring biomacromolecules or geomacromolecules including proteins, polysaccharides, and humic substances. | |
| Based on these possible nanomaterial transformations in the environment, the review further discusses knowledge gaps and measurement challenges as well as implications of nanomaterial transformations on efforts in environment, health and safety research. | |
| The authors conclude that transformations and the properties of the transformed nanomaterials will ultimately depend on the environment that they enter, and on the order of the environments in which they are exposed. This fact, and the uncertainty regarding use and release scenarios, makes the determination of risk challenging. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
