| Posted: Mar 18, 2013 | |
Plasmonic smart dust to probe local chemical reactions |
|
| (Nanowerk Spotlight) Conventional probing methods for localized surface properties often rely on ultra-high vacuum conditions. Consequently, approaches such as scanning tunneling microscopy, for example, have difficulties to resolve surface changes under realistic reaction conditions. Tip-enhanced Raman spectroscopy (TERS) can investigate arbitrary substrates and more diverse reaction environments but suffers from weak Raman scattering signals. Also, the fabrication of robust, reproducible, and highly enhancing tips is still challenging. | |
| Researchers have now presented a novel platform for the optical detection of localized chemical reactions on surfaces that can help overcome these difficulties by offering a sensitive, reliable, and easy-to-implement technique to probe local chemical reactions while they occur under diverse environmental conditions. This work has been published in the March 4, 2013 online edition of Nano Letters ("Plasmonic Smart Dust for Probing Local Chemical Reactions"). | |
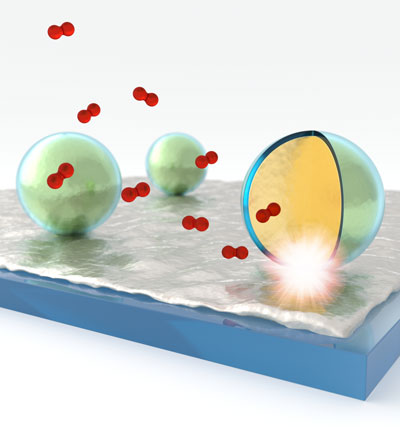
| Dubbed plasmonic smart dust, this all-optical platform consists of silica shell-isolated gold nanoparticles which can convert incident light into highly localized subdiffraction-limit hot-spots of the electromagnetic field and can thus optically report subtle environmental changes at their pinning sites on the probed surface. | |
 |
|
| Plasmonic probing of local chemical reactions. Smart dust (silica shell-isolated gold nanoparticles) are spread on a thin Pd film to locally probe the dissociation and subsequent uptake trajectory of hydrogen in Pd. (Image: Andreas Tittl, 4th Physics Institute, University of Stuttgart) | |
| "Our smart dust nanoparticles can be dispersed on many reactive and catalytically active surfaces to probe the local reaction kinetics in real-time and under realistic reaction conditions," says Harald Giessen, a professor at the 4th Physics Institute at the University of Stuttgart. "In our experiment, we apply this approach to the investigation of hydrogen dissociation and subsequent uptake in palladium thin films. Here, our platform can resolve subtle local environmental changes, both in the dielectric properties of the palladium film during hydrogen incorporation, as well as in its shape and morphology." | |
| Related work on plasmonic hydrogen sensing has been done, for example, using a nanofabricated plasmonic gold antenna to enhance the changes in a neighboring palladium nanoparticle. Also, arrays of palladium disks or single gold/palladium core-shell nanoparticles have been studied recently. | |
| "However, these approaches do not allow the localized detection of reaction kinetics on extended reactive or catalytic surfaces," says Andreas Tittl, a PhD student in Giessen's group and first author of the paper. "Thus, the key insight that led to the development of our sensor platform is that plasmonic smart dust particles can work as sensitive local reaction reporters, even when moving from ensemble to single-particle measurements." | |
| The combination of straightforward sensor chip fabrication via drop-coating and the strongly localized response of the smartdust nanoparticles leads to a versatile, label-free, real-time, and high-resolution platform for probing of local reaction kinetics. | |
 |
|
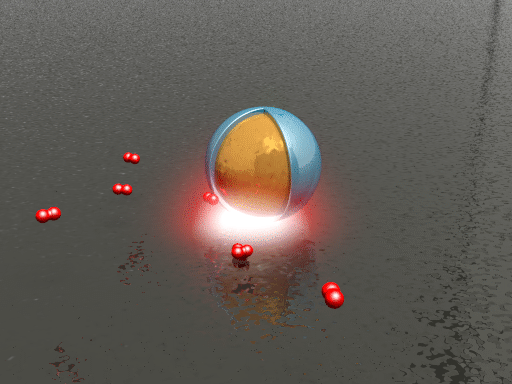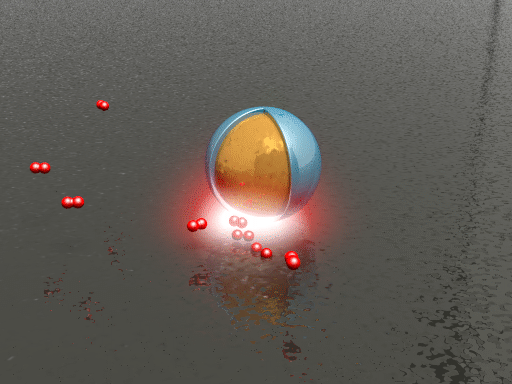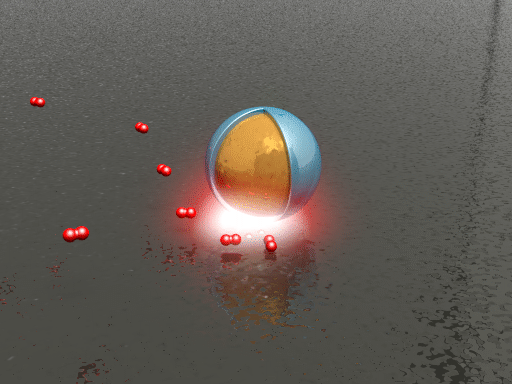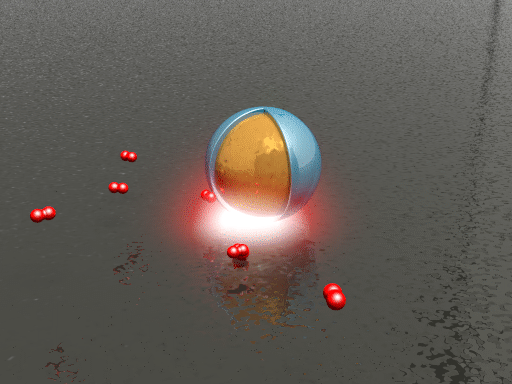
| Animation of the hydrogen dissociation and uptake on a palladium surface. The smart dust (a silica shell-isolated gold nanoparticle) reports changes in the local chemical environment via spectral shifts in its scattering spectrum. (Animation: Sven Hein, 4th Physics Institute, University of Stuttgart) | |
| To test the ability of their plasmonic smart dust for resolving different hydrogen contents in palladium, the researchers tracked hydrogen storage on palladium surfaces using the dust. They repeatedly exposed the sample to hydrogen concentrations ranging from 0.5 to 3% and found that different hydrogen concentrations can be clearly identified as intensity changes and spectral shifts of the plasmonic resonance with response times on the order of seconds. | |
| "The observed fast reaction kinetics compared to bulk palladium systems are due to the shorter diffusion lengths associated with palladium thin films and nanomaterials," says Tittl. "In general, higher hydrogen concentrations lead to increased resonance intensity and spectral blueshift of the resonance position." | |
| "One of the most interesting potential applications of our work is the possibility to produce a local reaction map by pinning multiple smart dust nanoparticles at reaction sites of interest and optically monitoring spatially distinct local chemical reactions simultaneously," notes Na Liu, group leader at the Max Planck Institute for Intelligent Systems in Stuttgart. "Especially, this approach can shed light on the influence of localized features such as cracks, facets, defects, and boundaries on the performance of reactive of catalytic materials." | |
| Furthermore, this method can be extended to investigate a plethora of chemical reactions on surfaces, ranging from the reduction and oxidation steps in fuel cells to catalytic water splitting. | |
| The team hopes that these exciting results will pave the way towards the development of inexpensive and high-output reaction sensors for real-world applications. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
