| Posted: Oct 18, 2007 | |
The unexpected benefits of using magnetic nanoparticles in immunoassays |
|
| (Nanowerk Spotlight) In old movies, saying "the rabbit died," was a popular way for a woman to reveal she was pregnant. The belief was that the doctor would inject the woman's urine into a rabbit. If the rabbit died, she was pregnant. The rabbit test actually originated with the discovery that the urine of a pregnant woman - which contains the hormone Human chorionic gonadotropin (hCG) - would cause corpora hemorrhagica in the ovaries of the rabbit. These swollen masses on the ovaries could only be detected by killing the rabbit in order to exam its ovaries. So, in reality, every rabbit died whether the woman was pregnant or not. Fortunately (for rabbits in particular), immunoassays - which can detect hormones (such as hCG), antibodies and antigens in the blood - were developed in the 1950s. Radioimmunoassays were first used to detect insulin in blood, but were later used for a variety of diagnostic tests. The technique is extremely sensitive and specific, but the necessary radioactive substances make it risky and expensive. In the 1960s, immunoassay technology was greatly enhanced by replacing radioisotopes with enzymes for color generation, which eliminated the risk and a great deal of expense. Today, most immunoassays are Enzyme-Linked ImmunoSorbent Assay, or ELISA. Because it can evaluate the presence of antigen or antibody in a sample, ELISA is commonly used to test for HIV, Hepatitis B, and West Nile Virus. ELISA has also been used in the food industry to detect potential food allergens such as milk, nuts, and eggs. Although there are numerous variations of ELISA, the test basically involves an antigen attached to a solid surface. When the antibody is washed over the surface, it will bind to the antigen. The antibody is then linked to an enzyme - usually a peroxidase (enzyme that causes oxidation) - which reacts with certain substrates, resulting in a change in color that serves a signal. The evolution of immunoassays has continued with developments such as fluorimetric immunoassay (which has replaced the rabbits in pregnancy tests.) Now, scientists at the Chinese Academy of Science have discovered a way to improve the process even more by eliminating one of the steps in certain immunoassays. | |
| "In order to increase sensitivity and decrease the noise of traditional biosensors, we have introduced a nanoparticle-based Sandwich ELISA into the system" Dr. Xiyun Yan explains to Nanowerk. "We did this by using two antibodies which were separately modified on golden and magnetic nanoparticles (MNPs). In general, iron oxide nanoparticles are considered to be inert, which is one of the characteristics that make them so useful for separation and imaging in biological systems. Binding iron oxide nanoparticles to metal catalysts or enzymes (such as peroxidases) gives them magnetic and catalytic properties." | |
| Yan, a professor at the National Laboratory of Biomacromolecules in the Institute of Biophysics of the Chinese Academy of Sciences (CAS) in Beijing, PR China, together with collaborators from CAS and Southeast University in Nanjing, shows that iron oxide nanoparticles are intrinsically active catalysts for oxidation reactions. The study has been published in the August 26, 2007 online edition of Nature Nanotechnology ("Intrinsic peroxidase-like activity of ferromagnetic nanoparticles"). | |
 |
|
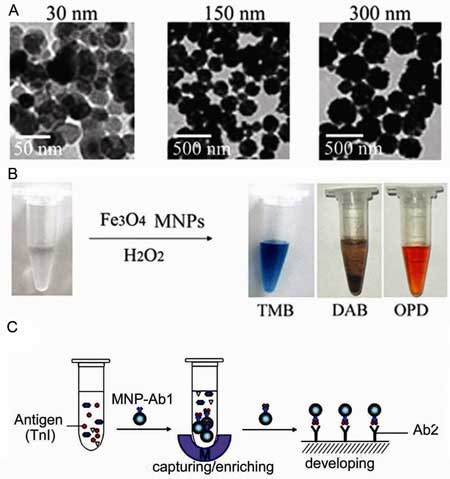
| Fe3O4 MNPs show peroxidase-like activity. A) TEM images of Fe3O4 MNPs of different sizes. B) The Fe3O4 MNPs catalyze oxidation of various peroxidase substrates in the presence of H2O2 to produce different color reactions. C) Immunoassays based on the peroxidase activity of Fe3O4 magnetic nanoparticles. (Image: Prof. Yan/CAS) | |
| "The magnetic properties allow separation of the target from a complex solution and the peroxidase assay can then be used to detect and quantify the amount of the target molecule" says Yan. According to the CAS scientists, Magnetic ELISA assays are already widely used for laboratory and diagnostic purposes. However, the difference when using the intrinsic activity of the MNPs compared to traditional ELISA, is that it cuts out the additional binding step with a secondary, enzyme-counjugated antibody. | |
| According to Yan, an MD with expertise in immunology, she stumbled upon the finding while designing a new type of biosensor for medical diagnostic purposes. As part of this process, Yan wanted to attach the antibody that recognizes her biological target to magnetic nanoparticles. To test whether she had successfully immobilized the antibody on the MNPs, she used a secondary antibody with Horseradish peroxidase attached as a detection tool. | |
| "In the process of doing control experiments, in which the peroxidase conjugated secondary antibody was omitted, we found that we could not get rid of the high background in the MNP assay, even if HRP was absent, and realized that the background HRP activity was coming from the MNPs themselves" says Yan. | |
| The CAS scientist recognized that this discovery was potentially exciting, but was unsure whether it made sense from a material and chemical point of view. She discussed her surprising findings with Prof. Sishen Xie, a renowned expert in nanomaterials. Xie cautioned Yan to very carefully confirm whether the peroxidase activity was really coming from the nanoparticles themselves, rather than being caused by contaminants of MNPs during their preparation. As he suggested, Yan then used nanoparticles from two different labs, each preparing them in different sizes and using different methods. Later, Yan contacted Dr. Sarah Perrett – a co-author of the paper – who has a chemical background, and together they investigated the mechanism of the peroxidase-like activity of the MNPs. | |
| The tests confirmed that the nanoparticles were active catalysts, and that the catalytic activity was greater in smaller particles. They also found that iron oxide nanoparticles are more stable over a variety of temperatures and varying levels of acidity, giving them an additional advantage over the traditional horseradish peroxidase. | |
| Yan and her collaborators have already developed two novel immunoassays (one for hepatitis B and the other for myocardial infarction) that exploit this new discovery. Using the appropriate antibody, the nanoparticles capture the target molecule in solution and then a magnet is used to isolate the nanoparticles. With the simple addition of a peroxidase-sensitive dye, the solution changes from clear to blue in the presence of the nanoparticles. | |
| According to the scientists, the stability, ease of production and versatility of the iron oxide nanoparticles makes them a powerful tool for a wide range of potential applications in medicine, biotechnology and environmental chemistry. | |
| "The MNP assay would be worth developing commercially, because it is cheaper, faster, more direct and potentially more sensitive," says Yan. "As a matter of fact, we are already collaborating with a company to develop the MNP-based immunoassay and to apply MNPs for the treatment of wastewater." | |
| In addition to the iron oxide nanoparticle catalyst activity, Yan says she has learned several other lessons from this study. "I think this study illustrates a couple of important points," she says. "One is that new and exciting discoveries often require an interdisciplinary approach – and the combination of both physical and biological knowledge. This study was involved cooperation between immunologists, protein chemists and physicists. This study also highlights the importance of following unexpected results, even when the result initially seems to be an annoying complication." | |
| By Cathy Garber, Copyright Nanowerk LLC | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
