| Posted: May 23, 2013 | |
Nanothermometers may aid in the treatment of cancer with heat |
|
| (Nanowerk Spotlight) Reporting in Nano Letters ("A Molecular Thermometer for Nanoparticles for Optical Hyperthermia"), a research team in Italy has developed gold nanoparticles coated with a temperature-sensitive dye. The constructs allow nanoparticle-induced heating to be monitored locally. | |
| “In the future, we see this technology being used clinically to monitor changes in temperature during nanoparticle-induced hyperthermia,” explains Dr. Giberto Chirico, Professor of Physics at the University of Milano-Bicocca. “But, the system also gives us a fundamental understanding of how irradiated nanoparticles transfer heat to their environment.” Chirico is co-lead author on the study together with his colleague Piersandro Pallavicini, professor of Chemistry at the University of Pavia. | |
| Hyperthermia refers to the treatment of cancer with heat. Because they are often poorly supplied with blood, cancerous tissues are more sensitive to increases in temperature compared to healthy tissues. Increasing the temperature of cancer cells beyond approximately 40°C is enough to induce programmed cell death, known as apoptosis. | |
| Hyperthermia has been used for decades to treat cancer. However, it is often difficult to apply heat locally to a tumor. Typically, limbs or other parts of the body that contain a tumor are heated externally. This often leads to considerable discomfort for the patient and damage to healthy tissues. | |
| Nanotechnology is helping to revolutionize cancer hyperthermia. Certain types of nanoparticles, in particular those made of gold or iron oxide, act as transducers that absorb electromagnetic radiation and generate heat. If the nanoparticles are delivered selectively to a tumour, heat can be generated within the tumour tissue by irradiating it with an external energy source. This results in heat-induced cell death within the tumor, sparing the surrounding healthy tissues. | |
| A number of studies have demonstrated that nanoparticle-induced hyperthermia is effective at killing cancer cells both in vitro and in vivo. Yet, one of the challenges facing the clinical application of this technology is the inability to monitor changes in the local temperature around the nanoparticles when an external energy source is applied. “Monitoring the local temperature is very important,” explains Chirico. “We want to heat the tissue to a high enough temperature to induce apoptosis in the cancerous cells, but not so high that we cause necrosis or damage healthy tissues.” | |
 |
|
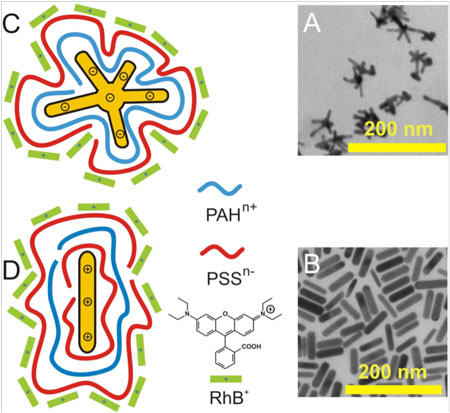
| Schematic showing the structure of the nanothermometers. A & B are electron microscopy images of the gold nanostars and nanorods (respectively). C & D are schematics of the gold nanostar and nanorod structures (respectively). The core gold nanoparticle is coated with alternating layers of charged polymers (red and blue). The final polymer layer is coated with rhodamine B (RhB) dye (green). Image reproduced, with permission, from the original article. (© 2013 American Chemical Society) | |
| Traditionally, local changes in temperature during hyperthermia were monitored using thermal imaging. While thermal imaging is sensitive, it has difficulty monitoring temperature changes that occur deep within tissues. Moreover, it has limited spatial resolution, making it difficult to pinpoint temperature changes within tissues. | |
| As an alternative to thermal imaging, tiny probes, known as ‘molecular thermometers’ can be used to monitor local changes in temperature. They typically operate based on temperature-dependent differences in the fluorescence intensity of a molecular dye. While these molecular thermometers are sensitive, and can measure changes in temperature with high spatial resolution, Chirico explains that they suffer from two major drawbacks. “First, the emission intensity of the dye is sensitive to the local environment. As a result, the thermometers need to be calibrated for each environment. Second, they are often separate from the nanoparticles used as thermal transducers. This means that you would need to administer different constructs and there is no guarantee you would be measuring temperature close to the nanoparticles.” | |
| Chirico and his team sought a way to monitor temperature changes using an integrated nanoscale construct that is not sensitive to the local environment. Their idea was to couple a fluroescent dye, Rhodamine B (RhB), to the surface of the nanoparticle. Instead of measuring the intensity of the fluorescence emission of the dye, they measured its ‘excited state lifetime’ (ESL). The ESL is the time it takes for the excited state of the dye to relax, with the emission of a photon of light. | |
| The researchers monitored the ESL of RhB as a function of temperature in a number of chemically distinct environments. “One of the most significant findings of this study was that, while the absolute value of the ESL is influenced by the surrounding environment, the dependence of the ESL on temperature is not,” explains Chirico. “This means that we do not need to calibrate the sensor for each environment.” The team found that the ESL of RhB showed a fundamental dependence on temperature of 0.029 ns/°C, which allowed them to convert a measured ESL for RhB into the local temperature around the nanoparticles. | |
| To examine differences in the heating profiles as a function of nanoparticle design, the researchers coupled RhB to three different types of nanoparticles that are commonly used as thermal transducers: gold nanorods, gold nanostars, and iron oxide nanoparticles. To avoid quenching the RhB fluorescence, the nanoparticles were coated with alternating layers of cationic and anionic polymers to act as spacers. The RhB molecules, which are positively charged at neutral pH, were electrostatically assembled to the polymer-coated nanoparticles. | |
 |
|
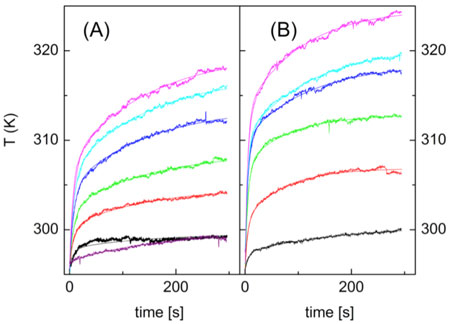
| Local temperature increases after laser excitation of A) gold nanorods, and B) gold nanostars. Temperature was measured under increasing laser power using the excited state lifetime of the coupled rhodamine B dye. Image reproduced, with permission, from the original article. (© 2013 American Chemical Society) | |
| The scientists then used the nanoparticles to measure changes in temperature as a function of time under constant excitation. They found that the temperature around the nanoparticles followed a bi-exponential relationship with two rate constants. The first constant describes how quickly the nanoparticles transfer heat to their surroundings, while the second constant describes how quickly the transferred heat is equilibrated. The first constant depends on the nature of the nanoparticles, while the second term depends on the environment. Gold nanostars were most efficient at transferring incident irradiation to the environment, followed by the gold nanorods, and then the magnetic nanoparticles. | |
| “Fundamentally, we can use the sensor to monitor how different nanoparticle designs change the heating profiles. This allows us to optimize the nanoparticle design for a particular application, or to monitor how exposing the nanoparticles to different environments changes the heating profile,” explains Chirico. | |
| Moving forward, Chirico and Pallavicini have fundamental questions to answer. First, they want to know how changing the symmetry of the nanostars changes the heat distribution profile. Second, they want to see whether the independence of the change in ESL as a function of temperature is a general principle that holds for other dyes in addition to RhB. “If it is a general phenomenon, we could substitute near-infrared dyes for RhB to monitor temperature changes deeper in tissues,” adds Chirico. | |
| Chirico, in collaboration with cancer institutes in Rome and Heidelberg, is also examining the efficacy of the technology in preclinical applications “We’re looking at applying these nanoscale thermometers in a mouse model of breast cancer and in cells in vitro. We want to see whether tracking temperature changes in real time can help us more effectively treat cancer with heat.” | |
| The nanothermometers developed by Chirico and his colleagues will allow particle-induced hyperthermia to be applied with greater specificity while avoiding damage to healthy tissues, helping to bring this technology one step closer to a clinical reality. | |
|
By Carl Walkey, Integrated Nanotechnology & Biomedical Sciences Laboratory, University of Toronto, Canada.
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
