| Posted: Jun 25, 2013 | |
Nanoscale chemistry allows microfluidics without channels and troughs |
|
| (Nanowerk Spotlight) In a seminal paper in Science more than 20 years ago, scientists described a device that was capable of causing drops of water placed on it to move uphill ("How to Make Water Run Uphill"). | |
| However, as it turned out in subsequent research, drops of water are notoriously difficult to move from where they lie, unless they are large enough to be moved by gravity, as happens with rain drops on window panes. For instance, two research reports ("Macroscopic transport by synthetic molecular machines" and "Light-Driven Motion of Liquids on a Photoresponsive Surface"), showed that water was one of the fluids where drops stubbornly refused to cooperate and move when cleverly engineered surfaces were activated to produce motion of fluidic droplets. | |
| In the absence of a microtube or of a channel – as they are required by most microfluidic devices – it usually is not possible to apply the pressure needed to induce liquid movement. An alternative approach, developed by researchers in Italy, is to pattern a gradient on a surface which allows a droplet to move in order to minimize its free energy. | |
| The novel method, reported in the June 14, 2013, online edition of Advanced Functional Materials ("And Yet it Moves! Microfluidics Without Channels and Troughs"), is simple and easily scalable to many drops and/or bigger drops. The work also provides a deep theoretical understanding of the phenomenon, which provides guidelines to improve the fabrication technique and improve the performance of this simple device. | |
 |
|
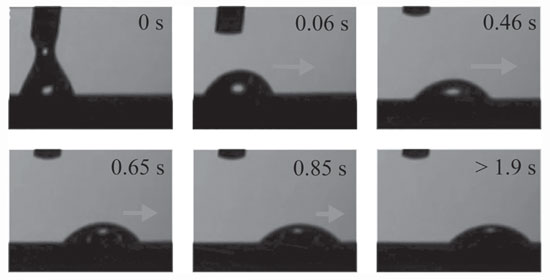
| Images of the droplet in motion. The arrows are proportional to the velocity of the movement. (Reprinted with permission from Wiley-VCH Verlag) | |
| "In our work, a drop of water is driven by surface forces against gravity and more importantly, in this case, against two competing phenomena: adhesion that would want the drop to stick to the surface where it is deposited and wetting that would want it to spread over it," Francesco Zerbetto, a professor in the Department of Chemistry at the University of Bologna, tells Nanowerk. "The power behind the self-propelling of these droplets is a simple trick: It's chemistry working at the nanoscale," adds Massimiliano Cavallini from the Institute of Nanostructured Materials, CNR Bologna, who, together with Zerbetto, led the work. | |
| Since liquid droplets move on horizontal surfaces only in the presence of a surface gradient – and the gradient must be sufficiently small to avoid both full wetting of the surface and pinning of the drop – the challenge is to properly prepare the wettability of the surface. | |
| "Two of the main approaches to modify the wettability of a surface leverage on the introduction of chemical heterogeneities on the surface and modification of surface topography by the creation of patterned or textured micro- or nanoscale features," explains Zerbetto. "In our work, we used the immersion method to prepare gradients of alkanethiols self-assembled monolayers (SAMs) in combination with a silicon surface functionalized by a highly hydrophobic molecule (TPOS). Optimization of the procedure allows us to demonstrate droplet motion." | |
| Depositing a droplet of water on the functionalized surface, the researchers observed directional movement of water droplets along the gradient without any external stimulus for contact angle slopes greater than 10°/mm. The drop displacement was 2.8 ± 0.1 mm in the direction of the more hydrophilic area. | |
| The ultimate goal of this research is to create a real microfluidic device – without the need of channels – where droplets can move and transport payloads and/or reagents along precise trajectories. To date, spontaneous movement without any external help is achieved using thermal gradients, sonication, etc. and is limited to a few millimeters. The challenge is to increase this distance to centimeters. | |
| Delivery is also one of the underlying issues at the basis of the work. Exploitation of the protocol proposed and demonstrated by Zerbetto and his team can deliver small quantities of water to specific locations, even when there are forces opposing such delivery, as in the case of hydrophobic conditions. | |
| Practical applications of this work are self-cleaning surfaces where drops of water carry away pollutants that are deposited on them. Imagine solar panels, lenses and mirrors that stay clean because self-propelled water drops scrub of the dirt. | |
| A self-propelled drop of water can also be the carrier of drugs or medication. "One could also think of situations where the molecule to be delivered is highly unstable, but can be produced by reacting two molecules that are brought together by means of two drops moving towards each other," says Zerbetto. | |
| So far, the scientists have demonstrated that their approach is able to produce directional drop movement. This result was obtained on a surface. In future applications, they will provide more complicated paths to span complex trajectories. | |
| "As is often the case, make it bigger, make it cheaper, make it faster, and make a lot of it is where we will go next," Zerbetto describes the team's goals. "In particular, make it bio-compatible and demonstrate that the whole thing can be inserted in a biological system will be one of our aims." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
