| Posted: Aug 13, 2013 | |
Gaps in U.S. nanotechnology regulatory oversight |
|
| (Nanowerk Spotlight) Life cycle assessments are essential tools for ensuring the safe, responsible, and sustainable commercialization of a new technology. Such an assessment takes a cradle-to-grave look at the health and environmental impact of a material, chemical, or product. | |
| With missing data about the large scale impact of nanotechnology, life cycle assessments of potential nanoproducts should form an integral part of nanotechnology research at early stages of decision making as it can help in the screening of different process alternatives. So far, however, life cycle studies of emerging nanotechnologies have been susceptible to huge uncertainties due to issues of data quality and the rapidly evolving nature of the production processes (read more: "Evaluation of 'green' nanotechnology requires a full life cycle assessment"). | |
| A recent paper ("From Cradle-to-Grave at the Nanoscale: Gaps in U.S. Regulatory Oversight along the Nanomaterial Life Cycle") from the Institute for Resources, Environment and Sustainability (IRES) at the University of British Columbia, investigates the suitability of the U.S. regulatory system as a comprehensive package addressing multiple types and uses of engineered nanomaterials (ENMs) over their life cycle. | |
| Specifically, the analysis, led by Terre Satterfield, Professor of Culture, Risk and the Environment and Director of IRES, examines whether current regulatory regimes are designed to trigger formal risk reviews for novel nanomaterials at each life cycle stage, and whether regulatory agencies have the appropriate tools, resources, and authority to manage potential risks. | |
| In their paper, the team reviews each regulation in the context of the collection of environmental, health, and safety (EHS) regulations to understand how existing shortcomings and new challenges posed by ENMs create gaps both within each regulation, and collectively over the entire set of regulations. | |
| There are basically three life cycle stages where various U.S. agenices – the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA), the Occupational Safety and Health Administration (OSHA), and the Consumer Product Safety Commission (CPSC) – could become active. The authors describe this in detail: | |
| "The first stage of the product life cycle includes ENM production, from the transformation of raw materials into nanomaterials (e.g., manufacturing bulk silver into nanoscale silver particles) to the incorporation of nanomaterials as a component of other products (e.g., nanosilver antimicrobial textile coatings). | |
| "Three key statutes that come into effect at this stage depend on the intended application of the nanomaterial. Chemical substances and pesticides are regulated under the Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), respectively, and the EPA administers both acts. Food additives and drugs are regulated under the Federal Food, Drug, and Cosmetic Act (FFDCA), which is administered by the FDA. Together, TSCA, FIFRA, and FFDCA apply to chemical substances, pesticides, food additives, and drugs primarily through a “premarket” risk-assessment, registration, and management approach. With this approach, each substance is evaluated and risk-management decisions are typically made before a product is released for use on the market. | |
| "The second stage of the product life cycle is the point at which a material or product is marketed and sold, and its intended function realized. At this stage, ENMs may be a component of a number of products or technologies including cleaning products, clothing, food packaging, electronic devices, and sports equipment. The FFDCA applies solely to dietary supplements and cosmetics at this stage. All other consumer products are regulated under the Consumer Product Safety Act (CPSA), which is enforced by the Consumer Product Safety Commission (CPSC). Regulations for supplements and cosmetics under FFDCA, and for consumer products under the CPSA operate through “postmarket” mechanisms whereby producers are responsible for reviewing and ensuring the safety of products, and regulatory agencies have the ability remove products from the market that are demonstrably “unsafe”. | |
| "The third stage represents the end point of a product life cycle wherein the nanomaterial is reclaimed for use in a new product, is destroyed by incineration, or is otherwise disposed. At this stage ENMs may already be incorporated into consumer products that have reached the end of their useful life, or may constitute a byproduct solid or liquid waste from industrial or consumer use. One main waste-related regulation comes into play at this stage: the Resource Conservation and Recovery Act (RCRA). Under RCRA, hazardous wastes must be tracked from their initial production to the time of their final disposition to ensure they are handled and disposed of safely. While an additional statute, the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) may also apply to waste, it deals with releases not otherwise controlled under RCRA. | |
| "Finally, releases of nanomaterials in occupational settings or into air, water, or soil can occur at any stage of the life cycle. The three statutes that apply across all stages of an ENM’s life cycle are the Occupational Safety and Health Act (OSHAct), the Clean Air Act (CAA), and the Clean Water Act (CWA), administered by OSHA and the EPA, respectively. Under the OSHAct, workplaces must ensure that exposure levels for hazardous materials do not exceed “permissible exposure limits” (PELs). The CWA and CAA are “end-of-pipe” statutes, and aim to prevent and control discharges of pollutants. The EPA is charged with setting standards for pollutant levels in ambient emissions, and regulates site-specific releases into the air and water based on facility-control permits." | |
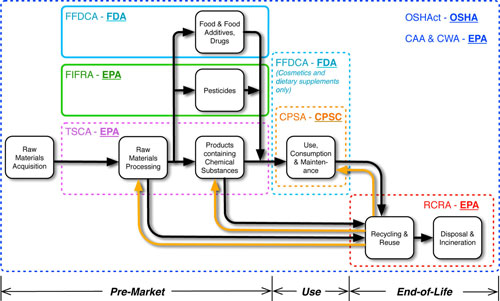 |
|
| Federal EHS regulations over the nanomaterial life cycle considering regulatory challenges posed by ENMs. Colored outlines highlight the primary points for risk review and risk management decision-making under each regulation (CAA, Clean Air Act; CPSA, Consumer Product Safety Act; CWA, Clean Water Act; FFDCA, Federal Food, Drug, and Cosmetic Act; FIFRA, Federal Insecticide, Fungicide, and Rodenticide Act; OSH Act, Occupational Safety and Health Act; RCRA, Resource Conservation and Recovery Act; TSCA, Toxic Substances Control Act). Regulatory agencies responsible for enforcing these regulations are highlighted in bold text (CPSC, Consumer Product Safety Commission; EPA, Environmental Protection Agency; FDA; Food and Drug Administration; OSHA, Occupational Safety and Health Administration). Solid outlines indicate regulations that are expected to enable comprehensive risk review and risk management for ENMs (FIFRA and FFDCA only). Dotted outlines indicate regulations that are not expected to enable agencies to comprehensively review risks or impose measures to manage risks from ENMs (Including CAA, CPSA, CWA, FFDCA for cosmetics and supplements, OSHAct, RCRA, and TSCA). (Reprinted with permission from American Chemical Society) (click image to enlarge) | |
| Having identified all the relevant statutes that come into play during the life cycle of nanomaterials, the authors then set out to assess the gaps in these regulations. They point out that U.S. Federal regulations are complex and nuanced, and the rapidly evolving regulatory environment makes evaluation of regulatory coverage for ENMs difficult. This is further complicated by nanomaterial behaviors and potential health and environmental implications, which are difficult to predict using conventional analytic tools. The authors then go on to describe some of the numerous gaps through which ENMs can avoid comprehensive risk review and federally mandated risk management measures. | |
| Engineered nanomaterials versus vonventional materials: In contrast to conventional materials for which regulators have a suite of tools to predict risks from chemical composition alone, not enough is known about the relationship between nanomaterial physicochemical characteristics and behavior to anticipate risks. Furthermore, without adequate ENM product information, reliable modeling tools, and standardized measures for risk analysis, proactive risk assessment at the premarket stage under TSCA, FIFRA, or FFDCA is difficult. This further complicated by the fact that ENMs present potentially more complex behavior in the environment than conventional materials due to a large number of intrinsic and extrinsic (environmental) variables that influence transformation, fate, transport, and toxicity. | |
| Exemptions and thresholds: Across the nanomaterial life cycle, several federal EHS regulations include applicability thresholds or provide mass-, volume-, or category-based exemptions that may allow some ENMs to escape federal oversight. A second point of contention under TSCA is the Low Volume Exemption (LVE) for substances manufactured/ imported in amounts less than 10 000 kg annually. A similar category-based exemption exists at the premarket stage for food additives under FFDCA. Premarket approval is not required if a food additive is “generally recognized as safe” (GRAS), a determination made by the manufacturer rather than the FDA. | |
| Data, uncertainty, and the burden of proof: The burden of proof for demonstration of risk rests with federal regulatory agencies rather than manufacturers (drugs, food additives, and pesticides excepted). Agencies generally operate on the principle of “safe until proven harmful”, which limits their options under conditions of high uncertainty. | |
| The authors summarize the following implications for nanomaterials oversight from the above gaps: "It is clear that under the current system a number of nanoproducts will be exempt from regulations, will not trigger thresholds for applicability, and may not be managed until they are categorized as hazardous (under RCRA, OSHAct), determined to be unsafe (CPSA, FFDCA), or can be specifically monitored and controlled (CWA/CAA). For those ENMs that do undergo assessment, limitations in existing models and methods and a general lack of risk-relevant data limit the scope and depth of the risk review. Further, many of these materials will undergo a one-time assessment, with no automatic re-evaluation when new information becomes available and our understanding of nanomaterial behavior improves. As a result, a multitude of nanomaterial products will make it through their life from cradle-to-grave with minimal regulatory oversight." | |
| Satterfield and her team cocnlude their paper with a number of suggestions on how to close these gaps. These include regulatory action, voluntary approaches to fill data gaps, increased funding for risk research studies, and the removal of barriers to information sharing, and enhancements in interagency regulatory coordination. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
