| Posted: Dec 02, 2013 | |
Microscale garbage truck cleans polluted water |
|
| (Nanowerk Spotlight) The construction of artificial micro- and nanomotors is a high priority in the nanotechnology field owing to their great potential for diverse potential applications, ranging from targeted drug delivery, on-chip diagnostics and biosensing, or pumping of fluids at the microscale to environmental remediation. Particular attention has been given to self-propelled chemically-powered micro/nanoscale motors, such as catalytic nanowires (read more: "Another nanotechnology step towards 'Fantastic Voyage'"), microtube engines (read more: "Microbots transport, assemble and deliver micro- and nanoscale objects") or spherical Janus microparticles (read more: "Novel motor system powered by polymerization"). | |
| In new work, researchers in Germany have now reported the first example of micromotors for the active degradation of organic pollutants in solution. The novelty of this work lies in the synergy between internal and external functionality of the micromotors. | |
| "Previously, some groups tried to demonstrate the use of catalytic nanomotors for biomedical applications – including ours – on-chip biosensors and capture of bio species," Dr. Samuel Sánchez, Group Leader Smart Nano-Bio-Systems, Max Planck Institute for Intelligent Systems, tells Nanowerk. "However, the toxicity of the fuel employed still limits their real applications. We imagined that environmental applications might be another field to explore, where the use of hydrogen peroxide is not controversial." | |
| In that direction, Wang’s group reported the removal of oil droplets ("'Microsubmarines' designed to help clean up oil spills") from solution, not degrading them. Now, Sánchez and his collaborators went one step beyond that and demonstrated the total removal of contaminants using micromotors. Indeed, the chemical is used for the self-propulsion and for the remediation when it interacts with the outer layer of the micromachine. | |
| "We have demonstrated the ability of self-propelled micromotors to oxidize organic pollutants in aqueous solutions through a Fenton process," explains Sánchez. "The combination of mixing and releasing iron ions in liquids results in a rate of removal of model pollutant (rhodamine 6G) ca. 12 times higher than when the Fenton oxidation process is carried out with nonpropelling metallic iron tubes." | |
| Reporting their results in The November 1, 2013 online edition of ACS Nano ("Self-Propelled Micromotors for Cleaning Polluted Water"), the research team from Max Planck, the Leibniz Institute for Solid State and Materials Research Dresden, and the Chemnitz University of Technology, demonstrates that micromotors boost the Fenton oxidation process (read more about Fenton reactions at the bottom of this article) without applying external energy, and complete degradation of organic pollutants is achieved. | |
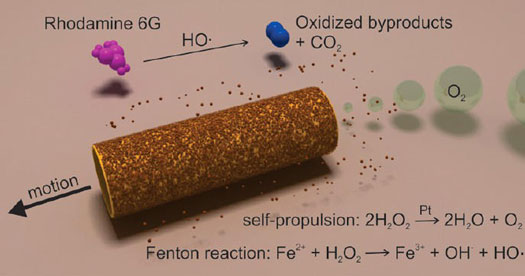 |
|
| Schematic process for the degradation of polluted water (rhodamine 6G as model contaminant) into inorganic products by multifunctional micromotors. The self propulsion is achieved by the catalytic inner layer (Pt), which provides the motion of the micromotors in H2O2 solutions. The remediation of polluted water is achieved by the combination of Fe2+ ions with peroxide, generating OH• radicals, which degrade organic pollutants. (Reprinted with permission from American Chemical Society) | |
| Sánchez notes that, if desired, the micromotors can be easily recovered using a magnet once the water purification process has been completed and the excess of hydrogen peroxide can be easily decomposed to pure water and oxygen under visible light. | |
| The team fabricated their tubular bubble-propelled micromotors containing small amounts of metallic iron (from 20 to 200 nm layer thickness) as outer layer and platinum as inner layer. The mechanism of degradation is based on Fenton reactions relying on spontaneous acidic corrosion of the iron metal surface of the micromotors in the presence of hydrogen peroxide, which acts both as a reagent for the Fenton reaction and as main fuel to propel the micromotors. Moreover, the ability of self-propelled, tubular micromotors to improve mixing results in a synergetic effect that enhances water remediation without applying external energy. | |
| This work can pave the way for the use of multifunctional micromotors for environmental applications where the use of hydrogen peroxide is not a major drawback but a co-reagent. | |
| Sánchez adds that the high efficiency of the oxidation of organic pollutants achieved by the Fe/Pt catalytic micromotors reported in this work is of importance for the design of new and faster water treatments, such as the decontamination of organic compounds in wastewaters and industrial effluents. | |
| The aim of this study was to fabricate an autonomous microscopic cleaning system that is working without external energy input in a much faster and convenient way. The micromotors offer this ability to move the catalyst around without external actuation or addition of catalysts (iron salts) to achieve water remediation, removal of organic dyes, etc. | |
| However, as the researchers point out, this is an application especially for microscale environments. "It is, unfortunately, clear that we would not use the micromotors in a large reactor vessel to clean huge amounts of water," says Sánchez. | |
| Nevertheless, the high efficiency of the oxidation of organic pollutants achieved by the Fe/Pt catalytic micromotors reported here is of importance for the design of new and faster water treatments, such as the decontamination of organic compounds in wastewaters and industrial effluents. | |
| "We have proven that the usefulness of the micromotors lies not solely in their capacity to move, but to exploit their motion using their external surface to enhance useful catalytic reactions," says Sánchez. "This work could open a new research line towards coupling a variety of catalytic reactions in self-propelled devices where the presence of hydrogen peroxide is not a disadvantage. We expect that a rich variety of contaminants can be in the next years be cleaned." | |
| Watch the micromotor in action. A synergetic effect is achieved taking advantage of the release of the iron ions from the outer layer of the micromotors and their active motion in the solution. | |
| About Fenton reactions | |
| The Fenton method is one of the most popular advanced oxidation processes for the degradation of organic pollutants, utilizing the hydroxyl radical (OH•) as its main oxidizing agent. The generation of OH• in the Fenton method occurs by reaction of H2O2 in the presence of Fe(II). However, one disadvantage of these processes is that Fe ions in solution must be removed after the treatment to meet regulations for drinking water. In order to diminish and, in the best scenario, solve the problems caused by the presence of Fe ions in treated effluents and decrease the costs of recovery, the use of heterogeneous Fenton catalysts is a promising strategy that could allow for the degradation of pollutants by Fenton processes without the requirement of dissolved iron salts. The micromotors fabricated in this work can be included as a new type of heterogeneous Fenton catalyst. With this method the remaining iron in the solution is one to three orders of magnitude lower than in conventional Fenton processes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
