| Posted: Jan 27, 2015 | |
Nanowaste - Nanomaterial-containing products at the end of their life cycle (page 3 of 4) |
|
| Use of nanomaterials in products | |
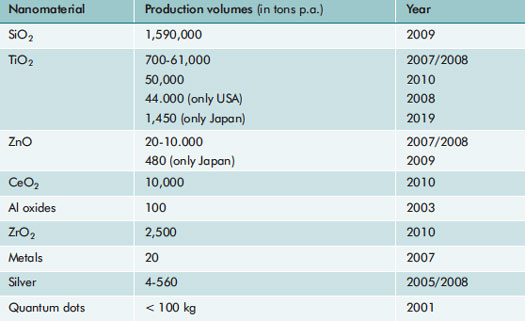
| Various nanomaterials are increasingly being employed in products for industry, trade, and consumers. The online database of the American Woodrow Wilson Center currently encompasses 1798 “nanoproducts” that are available on the international market.17 A study on the Austrian market identified 450 “nanoproducts” between late 2007 and April 2009. The most entries are in the categories “cosmetics” and “textiles”, which according to voluntary manufacturer information contain a number of nanomaterials (see18). The actually incorporated amounts of nanomaterials are unknown. Estimates of production volumes are available, but these often differ considerably from one another (Tab. 1). Accordingly, SiO2 and TiO2 are the nanomaterials with the highest production volumes worldwide and are probably the most common in products as well. | |
 |
|
| Table 1: Estimated global production volumes of different nanomaterials.19 | |
| Several past NanoTrust Dossiers have dealt with the various applications of nanomaterials. The following therefore only briefly summarizes several product categories. | |
| Textiles, for example for the outdoor sector, socks and underwear, can be impregnated with nanosilver to achieve an antimicrobial effect. Impregnation with nanoparticulate TiO2 or ZnO acts as a UV filter, and SiO2 has dirt- and water-repellent properties (see20). When these textiles are washed, nanomaterials can collect in sewage plants via wastewater. Studies on the behavior of nanomaterials during the washing process are available only for silver, whereby the released amounts vary considerably (see21). | |
| If textiles are chemically cleaned, then the produced wastes, including lint, can also contain nanomaterials. No studies have been conducted on nanoparticle releases during this process.22 Although used clothing is collected separately in Austria, nanomaterialcontaining textiles are also partially disposed of via the municipal solid waste stream in incineration plants. | |
| Cosmetics can contain carbon black (black pigment), TiO2, ZnO, SiO2 in their nano-form, or in certain cases also fullerenes as radical scavengers. These nanomaterials can enter household waste in incompletely emptied canisters and then be combusted in waste incineration plants, or enter wastewater during washing or showering. In particular, the nanoparticulate UV filter TiO2, which is a common component in sunscreen lotions (see23), can be released into the water when people bath or swim. No information is available on the exact amounts released, and only few studies have been conducted on potential negative ecological effects; these studies provide inconclusive evidence (see24). | |
| In paints and varnishes, nanosilver, TiO2, ZnO, SiO2 or Al2O3 (aluminum oxide) are used as biocides, as UV protection or to improve scratch- and abrasion resistance (see25). | |
| Containers with remnant contents are discarded as household waste, as hazardous waste or as construction wastes. Moreover, nanomaterials can also leach out of facade paint and enter the soil and waterbodies via rainwater, or enter sewage treatment plants through the sewer system.26 | |
| Cement-bound building materials (e.g. concrete) can also contain nanomaterials, for example SiO2 as aggregates to improve strength and stability, or TiO2 based on its photocatalytic “self-cleaning effect” or its ability to remove air pollutants (see27). | |
| Special sun protection glazing for windows or electrochromic (automatically adjusting) window glass to darken room interiors with nanoscale coatings of silver or wolfram oxide are still niche products in the construction business, but could find wider use in the future. Nanoparticles could be released with the dust produced when demolishing buildings. | |
| Such materials could also be released into the air during temporary storage, during the treatment process or when disposed in landfills. They could also enter the groundwater through wastewater or by leaching.28 No information is available on these processes. | |
| Nanomaterials composed of carbon, such as CNTs, fullerenes or graphene, are – according to manufacturer specifications – incorporated into the synthetics used in sports equipment. In tennis rackets, for example, they can help increase tensile strength (see29). | |
| When disposed of in household waste, the majority of such products is incinerated in Austria. CNTs most likely burn completely in such facilities, but it cannot be excluded that small fractions enter the environment through flume gases or solid residues (see30). In Austria, household wastes must be pre-treated, but in other countries they are directly disposed in landfills (compare Fig. 3). | |
| A study in the USA shows that CNTs – when these come into contact with other wastes in landfills – are partially bound or retain their stability.31 Leaching of CNTs and other “nano-wastes” depends strongly on the respective landfill conditions. No information is available on the behavior of fullerenes or graphene. | |
| Nanoparticulate crystals made of semiconductor materials, so-called quantum dots, are increasingly being applied in the electronics industry. Examples include modern televisions with LED backlighting but also the production of lamps (LED) and in highly efficient thin-film solar cells. Based on their potential content of arsenic, cadmium, europium, gallium, indium, tellurium, etc., LEDs should be collected as “problematic waste” (hazardous waste) and not be discarded in household garbage. | |
| The semiconductor gallium arsenide is particularly problematic due to the toxicity of arsenic. This is because, in the absence of atmospheric oxygen and water, a very thin oxide layer can form on the surface of the material; it is highly toxic and could damage the environment. | |
| Quantum dots can contain numerous rareearth metals, and resource policy considerations dictate that these should be recycled. | |
| The recycling processes, for example for LEDs, are currently still under development and very expensive.32 As provided for by the EU guide lines on waste electrical and electronic appliances, LEDs must be taken back by the traders or manufacturers and properly disposed of. | |
| Continue to next page (4 of 4) | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
