| Posted: Feb 05, 2008 | |
Nanostoves for superfast blood analysis and DNA defect detection |
|
| (Nanowerk Spotlight) Walk into an intensive care unit and you're likely to see many of the patients sporting a 'central line' - a plastic tube placed in a large vein that goes to the heart. A central line is a very efficient way to pump nutrients, antibiotics or other drugs directly into the bloodstream. | |
| Unfortunately it is also a cause for bloodstream infections (sepsis) and central lines remain an important cause for hospital deaths. An estimated 200,000 bloodstream infections occur each year in the U.S. alone, the majority associated with the presence of an intravascular catheter. | |
| The Institute for Healthcare Improvement (IHI) estimates the yearly death toll from blood infections related to intravenous lines to be as high as 28,000. Numerous pathogens can cause sepsis and the death toll could be reduced if the specific pathogen could be identified more quickly than it is usually done today using blood cultures (the laboratory examination of a blood sample to detect the presence of disease-causing microorganisms). | |
| One solution would be to determine the pathogen's DNA, requiring a rapid DNA assay with the potential for mutant identification and multiplexing. Current DNA assays are based on thermal dehybridization or melting of the DNA duplex helix – the melting temperature of DNA is determined by its base sequence – a process that can take up to an hour. | |
| Researchers in Germany have developed a novel technique that allows for DNA analysis in the millisecond range. Their method has great potential to vastly improve the speed of pathogen detection. | |
| Apart from the 'frontline' blood analysis requirements in hospital environments, DNA analysis is a huge and growing field in the area of DNA defect analysis for instance in researching hereditary diseases. Many diseases are caused by defective DNA sequences. One of the side effects of these defects is that they reduce the melting temperature of the DNA (in defect locations the two DNA strands don't match perfectly and therefore separate at lower temperatures than are required to separate matching base pairs). Researchers use this to identify DNA defects by measuring its melting curve. | |
| The way this has been done so far is to combine DNA sequences with gold nanoparticles and then to slowly, usually in a water bath, increase the temperature of the DNA-containing solution. During the heating process the optical absorption is monitored by laser. Aggregates consisting of gold nanoparticles linked via DNA are changing their absorption properties when the DNA strands dehybridize, since this leads to a break-up of the entire aggregate. The melting temperature has been determined by gently heating the entire solution always keeping the entire system in thermodynamical equilibrium. Since this is a protracted process, taking up to one hour, it is not applicable in high-throughput DNA analyses. | |
| "So far, laser induced heating of gold nanoparticles has mainly been applied to destroy tissue or cell membranes" Prof. Jochen Feldmann explains to Nanowerk. "We have used gold nanoparticles as optically controllable and pulsed nanostoves. This local and temporally limited way of heating gold nanoparticles bears the possibility to induce thermally driven reactions on the nanoscale within a limited time window. Instead of waiting for equilibrium conditions on the macroscopic scale, we just measure the temporal behavior of the system on its natural nanoscale." | |
| Feldmann's group at the University of Munich, together with Roche Diagnostics and the group of Prof. Thomas Klar from the University of Ilmenau, all in Germany, have developed a technique that allows the millisecond fast analysis of DNA. In contrast to destructive applications of gold nanoparticle- mediated optical heating, the German researchers apply gold nanoparticles as nanoscopic stoves for gentle, controlled, and reversible heating. | |
 |
|
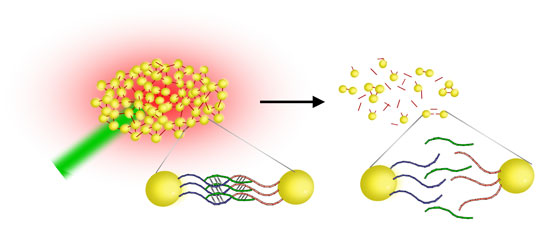
| Gold nanostoves as DNA melting assay. After the addition of the target to the functionalized gold nanoparticles, multiple interparticle connections form, resulting in stable DNA-nanoparticle networks. Heating the networks with a laser (green) results in a breakup of the networks once the melting temperature is reached (Image copyright: Prof. Jochen Feldmann, University of Munich) | |
| "This allows us for the first time to use gold nanoparticles for optically induced DNA melting" says Feldmann. "We use DNA-bound gold nanoparticles aggregates as light absorbers to locally convert optical energy from 300 ns laser pulses into thermal energy. This thermal energy is used to melt double-stranded DNA. Subsequently, the aggregates disintegrate on a millisecond time scale." | |
| In this technique, the aggregates of nanoparticles serve as both converters of optothermal energy to heat the DNA and as a spectral reporter of DNA melting that allows for the discrimination of different targets even when they are mixed in one and the same solution. | |
| Both, the perfectly matching and the single base pair mismatched DNA can be clearly distinguished even in a 1:1 mixture of both targets. The millisecond observation window provides the fastest time frame for a DNA melting analysis to date with a potential for multiplexing and mutant identification. | |
| With this new technique it is possible to adjust the intensity of the laser so that only defective DNA melts but not healthy one. This allows to check with a single, millisecond measurement if a sample of DNA is defective or not. Given its unprecedented speed, this novel technique allows for a new high-throughput analysis technology. | |
| Gold nanoparticles exhibit unique chemical and physical properties. They are non-toxic and can easily be attached to almost any macro- and biomolecule. They show unique optical and electrical properties. Apart from using them as optically controllable nanostoves they can act as nanosized amplifiers for spectroscopic signals such as fluorescence and Raman. They can act at the same time as nanosized manipulators, sensors, amplifiers and transmitters. This makes them highly interesting for many scientific and technological applications in material science, biotechnology, cell biology, pharmacy and medicine. | |
| Feldmann is convinced that his team's findings will stimulate further optothermal manipulation experiments on the nanoscale. "Many important molecular and biomolecular reactions are controlled or initiated by temperature" he says. "Attaching gold nanoparticles allows for external and optical control of such reactions. The non-destructive nature of our optothermal control method is clearly of great importance for in vivo thermal manipulation experiments." | |
| The researchers reported their findings in the January 28, 2008 online edition of Nano Letters ("Gold NanoStoves for Microsecond DNA Melting Analysis"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
