| Posted: Mar 05, 2008 | |
Nanotechnology's role in next generation biofuel production |
|
| (Nanowerk Spotlight) Years of engineering research and design, together with uncounted billions of dollars from government and industry, went into the development of the modern petroleum industry. It would be unreasonable to expect that we can replace this industry with greener alternatives without a similarly expansive and sustained effort. Point in case is a recently published roadmap to 'Next Generation Hydrocarbon Biorefineries' that outlines a number of novel process pathways for biofuels production based on scientific and engineering proofs of concept demonstrated in laboratories around the world. The key conclusion from this (U.S.-centric) report is that "while the U.S. has made a significant investment in technologies focusing on breaking the biological barriers to biofuels, principally ethanol, there has not been a commensurate investment in the research needed to break the chemical and engineering barriers to hydrocarbon fuels such as gasoline, diesel, and jet fuel." | |
| This statement of course holds true not only for biofuels but for any kind of green energy technology. The production of ethanol from corn (about 95% of ethanol in the U.S. is produced from field corn whereas Brazil, the world leader in ethanol production, uses sugar cane) has come under intense scrutiny and discussion for its potential environmental and economic side effects (see fore example Panel Sees Problems in Ethanol Production or read the UN report on sustainable bioenergy - pdf download, 1 MB). Advances in agriculture and biotechnology have made it possible to inexpensively produce lignocellulosic biomass (plant biomass that is composed of cellulose and lignin) at costs that are significantly lower (about $15 per barrel of oil energy equivalent) than crude oil. | |
| The key bottleneck for lignocellulosic-derived biofuels is the lack of technology for the efficient conversion of biomass into liquid fuels. Advances in nanotechnology have given us an unprecedented ability to understand and control chemistry at the molecular scale, which promises to accelerate the development of biomass-to-fuels production technologies. | |
| If you manage to ignore the sound of thundering herds of hypsters stampeding into their green (green as a dollar bill, that is) future you will find plenty of examples that science and technology, especially nanotechnology, is providing us with the capabilities to improve the design of materials, processes and applications that minimize hazard and waste. There are many examples of very specific opportunities here on the Nanowerk website; for instance, just a few days ago we ran a news story about how nanosieves save energy in biofuel production. | |
| Also, if you are interested, we have posted Nanowerk Spotlights on 'green' topics ranging from nanotechnology and water treatment, to clean hydrogen production or a more general overview on nanotechnology's potential to reduce greenhouse gases. | |
| The production of hydrocarbon fuels from biomass (biofuels) has become a big issue recently because they appear to offer a relatively short-term solution to replacing part of the petroleum-based fuels in our energy production with 'green' hydrocarbon fuels. These 'green' hydrocarbon fuels are essentially the same as those currently derived from petroleum, except that they are made from biomass. A word of caution: 'green' in this context does not necessarily mean that these fuels are better for the environment, although they often are, but rather than from 'black' fossil-based sources they come from renewable 'green' plant material. | |
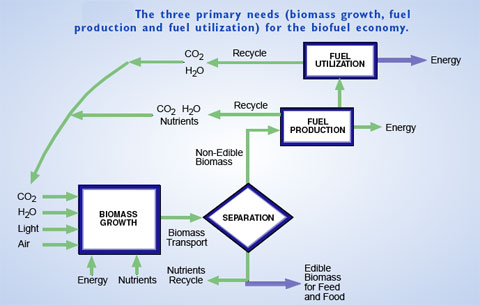 |
|
| (Source: Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Next Generation Hydrocarbon Biorefineries) | |
| A new report (in draft format) on biofuel production, titled "Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Next Generation Hydrocarbon Biorefineries" is based on the results from a June 2007 workshop and was issued by the University of Massachusetts Amherst. | |
| The purpose of the workshop was to articulate the essential role of chemistry, chemical catalysis, thermal processing, and engineering in the conversion of lignocellulosic biomass into green gasoline, green diesel and green jet fuel. | |
| Sponsored by the National Science Foundation and the Department of Energy, the workshop identified the basic research needs and opportunities in catalytic chemistry and materials science that underpin biomass conversion and fuel utilization, with a focus on new, emerging and scientifically challenging areas that have the potential for significant impact. The report illuminates the principal technological barriers and the underlying scientific limitations associated with efficient processing of biomass resources into finished fuels: "The limiting factor to biofuels production is simply that low-cost processing technologies to efficiently convert a large fraction of the lignocellulosic biomass energy into liquid fuels do not yet exist." | |
| The report is structured into six main chapters: | |
|
|
|
| Chapter six in particular deals with the design of recyclable, highly active and selective heterogeneous catalysts for biofuel production using advanced nanotechnology, synthesis methods and quantum chemical calculations. | |
| One sidebar in the report addresses the nanoscience of catalyst synthesis: "Calls are often heard to 'transform the art of catalyst preparation into a science.' One arena in which this is occurring is in fundamental studies of catalyst impregnation, the process by which a solution containing dissolved metal precursors is contacted with the high-surface-area catalyst support. In this vein of work, the chemical fundamentals of impregnation are being understood and exploited for simple, rational methods to make metal nanoparticles. Most industrial catalysts are of this sort and employ aluminum oxide or carbon as the support." It the shows an example of platinum/silica catalyst synthesis by strong electrostatic adsorption (SEA). | |
| Fundamental understanding of catalytic chemistry is obtained when the detailed, atomic scale structure of a catalyst and its chemical composition is known and can be correlated to its catalytic activity and selectivity. So far, the tools for in situ real-time monitoring of catalyst structure at the nanoscale are not very effective and the report urges for spectroscopic, scattering and imaging tools to be developed. For instance, next-generation electron microscopes that allow for imaging of nanometer sized features in thin liquid layers are needed to explore the structural changes of catalysts in aqueous environments. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
