| Mar 10, 2008 | |
Atomic Force Microscopy (AFM) - A key tool for nanotechnology |
|
Table of Contents
| |
IntroductionAtomic Force Microscopy (AFM) has become one of the most widely used tools for imaging, measuring, and manipulating matter at the nanoscale. Whenever you read an article about nano this or nano that, chances are you come across a large number of confusing three-letter acronyms – AFM, SFM, SEM, TEM, SPM, FIB, CNT and so on. It seems scientists earn extra kudos when they come up with a new three-letter combination. |
|
| One of the most important acronyms in nanotechnology is AFM – Atomic Force Microscopy. This instrument has inspired a variety of other scanning probe techniques and has found applications in numerous fields, including materials science, biology, and nanotechnology. AFM's ability to visualize nanoscale objects and measure various properties with high resolution and sensitivity has made it an essential tool in the development of nanosciences and nanotechnologies. | |
History & Background of AFM |
|
| The invention of the Scanning Tunneling Microscope (STM) in 1982 and the Atomic Force Microscope (AFM) in 1986 laid the foundation for the rapid growth of nanosciences and nanotechnologies over the past 20 years. These tools, combined with refined processes such as electron beam lithography, allowed scientists to deliberately manipulate and manufacture nanostructures, which was not possible before. | |
| The AFM's ability to measure the topography, mechanical, and electrical properties of a sample at the nanometer scale has led to its widespread use, with over 20,000 AFM-related papers published, over 500 patents issued, and approximately 10,000 commercial systems sold (not counting a significant number of home-built systems). | |
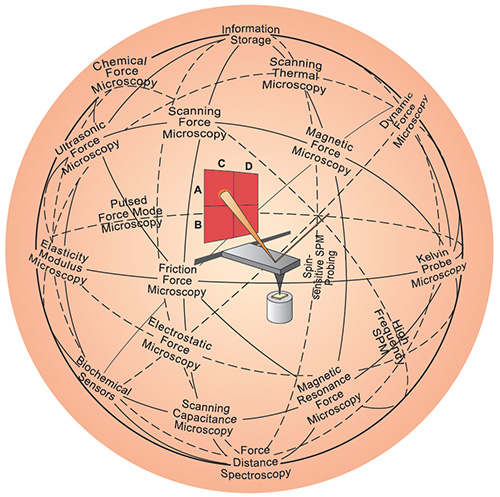 | |
| The AFM (center) has inspired a variety of other scanning probe techniques. Originally the AFM was used to image the topography of surfaces, but by modifying the tip it is possible to measure other quantities (for example, electric and magnetic properties, chemical potentials, friction and so on), and also to perform various types of spectroscopy and analysis. (Image: Christoph Gerber; © Nature Publishing Group) | |
AFM Working Principle |
|
| The term microscope in the name is actually a misnomer because the information is gathered by feeling the surface with a mechanical probe rather than by looking through lenses. Unlike other imaging techniques like x-ray diffraction, AFM does not use lenses, which means its resolution is not limited by the diffraction limit. | |
The operation principle of an AFM is based on three key elements:
|
|
AFM Components and Instrumentation |
|
The main components of an AFM include:
| |
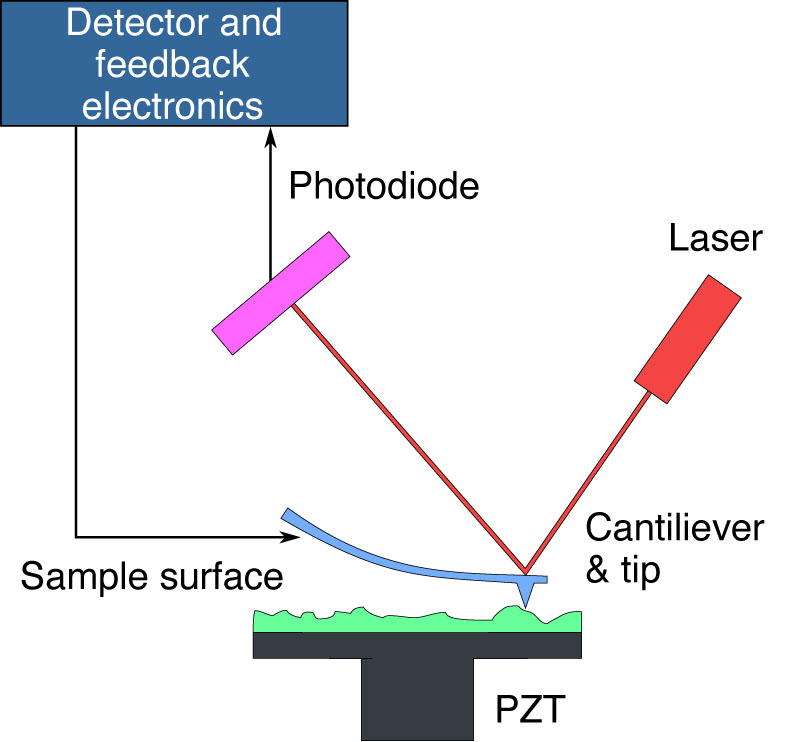 | |
| This schematic illustrates the functioning of an Atomic Force Microscope (AFM). An AFM uses a sharp tip on the end of a cantilever to scan the sample surface. As the tip encounters surface variations, the resulting deflection alters the laser beam's path, which is monitored by a photodiode. The system's detector and feedback electronics adjust the cantilever's height to keep the laser's reflection consistent, allowing for the reconstruction of the sample's topography by tracking the cantilever's position. The piezoelectric transducer (PZT) facilitates precise movement of the sample in three dimensions to enable detailed imaging. (Image: public domain) | |
AFM Modes Overview |
|
The AFM can be operated in a number of modes, depending on the application, but four modes are most commonly used for AFM imaging:
|
|
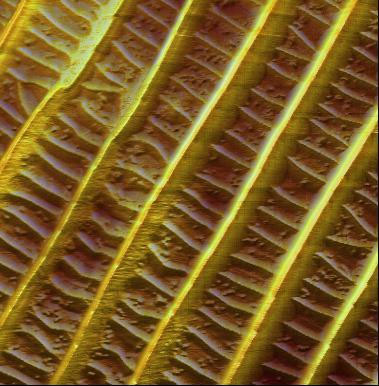 | |
| Butterfly wings are fragile biological nanostructures that derive their color both from the structure of the scale cells on their surface and from pigment grains. The image shows an AFM measurement of these optically invisible, fragile structures in dynamic operating mode (scan size: 10 µm, height range: 1.60 µm). (Image: Nanosurf) | |
Image Processing and Data Analysis |
|
AFM data can be processed and analyzed to extract valuable information about the sample's properties:
| |
Advanced Imaging Modes and Applications |
|
| An article in the Encyclopedia of Life Sciences, written by Martijn de Jager and John van Noort, both from the University of Leiden in The Netherlands, gives an excellent overview of Atomic Force Microscopy and its applications in life sciences. Below we are summarizing some of the key information from this article. | |
| As de Jager and van Noort write in their article, large numbers of various biological samples, including cells, cell compartments and biomolecules, have been studied with AFM. "In some of these studies, AFM is used as a plain imaging tool to investigate the topography of immobilized and/or fixed samples, complementing existing methods such as electron microscopy, with the advantage that sample preparation is generally more straightforward. | |
| For other experiments, the use of AFM is a prerequisite to look at nonfixed materials and even their dynamics in aqueous environment. Besides its imaging capabilities AFM is becoming increasingly important as a nanomanipulation tool. The single-molecule analysis of interaction forces, elasticity and tertiary protein structure in intact biological materials is uniquely possible using AFM." | |
| Let's take a look at two examples illustrated in the paper: | |
| Imaging cells | |
| "AFM imaging of living cells provides a direct measurement of cell morphology with nanometer resolution in three dimensions. Because of its noninvasive nature and the absence of fixation and staining, even dynamic processes like exocytosis, infection by virus particles and budding of enveloped viruses have been successfully visualized in successive scans. Owing to the high elasticity of the cell membrane, the tip can deeply indent the cell without disrupting the membrane. Making use of this effect, even submembraneous structures such as cytoskeletal elements or organelles like transport vesicles can be revealed. However, due to the elasticity of the cell the contact area between the tip and the sample increases with increasing applied force. The elastic modulus of living cells varies between 10 and 100 kPa, which results in a tip sample contact area of 50–100nm in the softest region of the cell. Therefore, the (sub-) nanometer resolution that is routinely achieved on more rigid samples cannot be achieved on membranes of intact cells." | |
| Structure, function and interaction of single DNA and protein molecules | |
| "Besides the analysis of cells and cell membranes, AFM-based methods to study purified single molecules like proteins, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) have developed rapidly in the past decade. Unique details on the mechanism and function of DNA- and RNA metabolizing proteins can directly be obtained by quantification of the number, position, volume and shape of protein molecules on their substrate. Like other single molecule techniques all individual instances of the entire population of structures are revealed, also showing rare but important species. Further insights in the mechanism of a reaction can be obtained from image analysis by measuring parameters such as protein-induced DNA bending, wrapping and looping. Besides topography imaging, force spectroscopy has been successful in unraveling tertiary structure in proteins, RNA and other polymers." | |
AFM has also been adapted to measure various other properties of the sample by modifying the tip and the scanning mode:
|
|
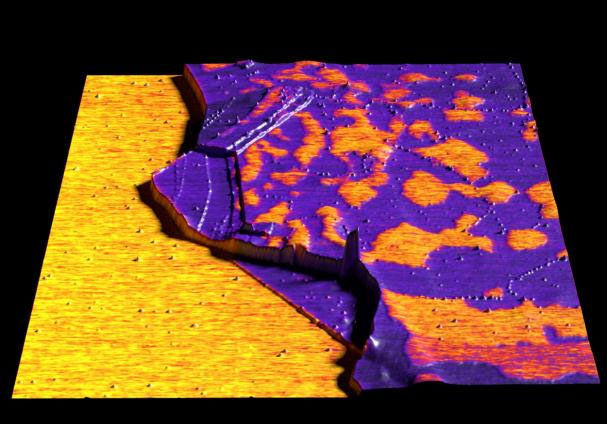 | |
| Graphene imaged in Kelvin probe force microscopy (KPFM) mode. (Image: Nanosurf) | |
Advantages and Limitations of AFM |
|
AFM offers several advantages over other nanoscale imaging techniques:
| |
However, AFM also has some limitations:
| |
Comparison with Other Nanoscale Imaging Techniques |
|
| AFM complements other nanoscale imaging techniques, such as Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). While SEM and TEM offer higher resolution and faster imaging speeds, they require special sample preparation and vacuum conditions. AFM, on the other hand, can image samples in their native environment and can measure various properties beyond topography. | |
Future Developments and Trends in AFM |
|
| Although it already is an essential tool for structural analysis and manipulation of complex macromolecules and living cells, it is to be expected that AFM-based applications will be further extended in the future. Technical developments will advance the AFM system itself, by improvement of resolution, image rate, sensitivity and functionality. A combination with complementary techniques will fill in some limitations of AFM. | |
| To fully exploit the potential of AFM to study functional biomolecules and their interactions, de Jager and van Noort say that video microscopy would be needed to capture dynamic events. "Currently, the scan rate is limited by the mechanical response of the cantilever and the piezo. Smaller cantilevers will result in higher resonance frequencies, allowing faster scanning rates. By reducing the size of the cantilevers one order of magnitude, the frame rate can be reduced from typically a minute down to video rate, allowing the study of a significantly larger range of biomolecular processes." | |
| The two researchers expect the most important developments for the tip itself. "Image resolution in all modes is dependent on tip geometry. The reduction of tip size, increase of its aspect ratio and its resistance to wear as a result of scanning will have a considerable impact on all AFM applications." | |
| For instance, researchers at Harvard and Stanford universities have developed a specially designed AFM cantilever tip, the torsional harmonic cantilever (THC), which offers orders of magnitude improvements in temporal resolution, spatial resolution, indentation and mechanical loading compared to conventional tools. With high operating speed, increased force sensitivity and excellent lateral resolution, this tool facilitates practical mapping of nanomechanical properties. | |
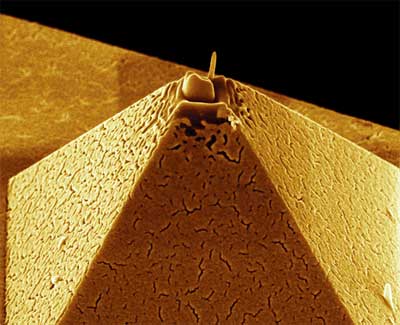 | |
| Developing new instruments to be able to "see" at the nanoscale is a research field in itself. Shown here is the tip of an AFM probe tip. Here, a platinum electrode has been deposited on the tip of this pyramid shaped AFM tip via focused ion beam (FIB) deposition. (Image: C. Menozzi, G.C. Gazzadi, S3 (INFM-CNR), Modena. Artwork: Lucia Covi) | |
| More beautiful images made with scanning probe microscopes are here. | |
|
And if you want to elevate your understanding and practical skills in AFM, from its foundational principles to advanced applications, check out this AFM Master Class with 8 Youtube tutorials. | |
Conclusion |
|
| Atomic Force Microscopy has revolutionized the field of nanoscale imaging and characterization. Its ability to measure various properties of a sample with high resolution and sensitivity has made it an indispensable tool in nanosciences and nanotechnologies. With ongoing advancements in instrumentation and methodology, AFM will continue to play a crucial role in unlocking the secrets of the nanoscale world. | |
| The development of AFM has not only enabled scientists to visualize and study nanoscale structures and phenomena but has also paved the way for the manipulation and fabrication of materials at the nanoscale. The impact of AFM extends far beyond the realm of basic research, with applications in fields such as materials science, electronics, medicine, and biotechnology. | |
| As the demand for nanoscale characterization and manipulation continues to grow, it is evident that AFM will remain at the forefront of nanotechnology research and development. The continuous improvement of AFM techniques and the integration of AFM with other complementary methods will further expand its capabilities and open up new avenues for scientific discovery and technological innovation. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
