| Posted: Mar 18, 2008 | |
Biohybrid nanocontainers with controlled permeability |
|
| (Nanowerk Spotlight) Each of the 100 trillion or so cells that make up your body is the ultimate molecular machine. Cells can take in nutrients, convert these nutrients into energy, carry out specialized functions, and reproduce as necessary. Each cell stores its own set of instructions for carrying out each of these activities. A cell, although measuring only 10 micrometers on average, is a hugely complex system. Researchers are not even coming close to being able to build artificial cells that duplicate their biological archetypes. That doesn't mean they are not trying. But before you can duplicate something you first need to understand how it works and what its components are. In the case of biological cells, that mostly means understanding the role of proteins which carry out the bulk of cellular activity, and the molecular networks in which they operate. | |
| To probe these processes, scientists have developed single-molecule imaging techniques that allow them to observe, measure, and manipulate some the key processes at the very core of life. Scanning probe techniques allow imaging of single molecules on surfaces, and specialized optical techniques enable their characterization in complex environments. Single molecule biomechanical studies have been used to manipulate individual molecules and to measure the force generated by molecular motors or covalent bonds. | |
| Understanding and manipulating cellular function at the level of individual molecules is within reach. One of the requirements of single molecule techniques is the ability to follow an individual molecule for sufficiently long times in solution. However, it is a challenge to cope with the effects of Brownian motion (the random motion of small particles suspended in a gas or liquid) on this time scale. To meet this challenge, more recently, biomolecules have been encapsulated inside lipid vesicles, which are themselves tethered to a surface. Now, a novel nanocontainer offers controlled permeability functionality which not only is desirable for single molecule imaging but also is a very important property for micro- and nanodevices and for delivery of drugs or imaging agents in vitro and in vivo. | |
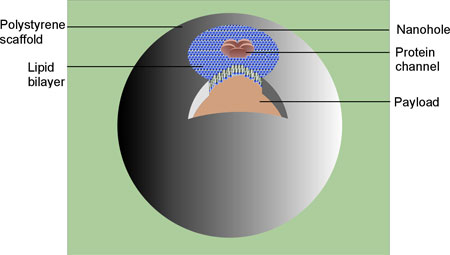
| By combining the advantages of both lipid and polymer containers, researchers in The Netherlands have generated an advanced bio-hybrid nanocontainer. The main components of this hybrid nanocontainer are the scaffold, which is made of a polymer; a lipid membrane patch that was sealed over a hole in the scaffold; and a remote-controlled channel protein, which is reconstituted in the lipid patch before sealing the hole. It allows the loading and unloading of a payload at a desired time and/or location. | |
| The channel protein chosen is the mechanosensitive channel of large conductance (MscL) from Escherichia coli. In nature, MscL works as a safety valve. Under severe hypoosmotic stress conditions, it responds to tension in the membrane by undergoing structural changes and forming a temporary pore in the membrane. Being incorpoaretd in an artificial nanovessel, this system offers the controlled communication between the interior and exterior of the vesicle containers. | |
 |
|
| Schematic representation of a hybrid nanocontainer, consisting of an impermeable hollow polymeric scaffold with a single small opening and a payload inside the scaffold. Synthetic bilayer lipid membrane with embedded MscL channels seals the hole. (Image: Dr. Dudia) | |
| "Vesicles serve as a biologically relevant environment, where reactions occur in small compartments, and it provides freedom to the molecules, as long as they are not interacting with the vesicle walls" Dr. Alma Dudia explains to Nanowerk. "The major disadvantage of vesicle encapsulation, on the other hand, is the difficulty to exchange the substances/ solutions between the interior and the exterior of the vesicles due to the lipid bilayer barrier. Our approach provides control over the permeability of molecules, achieved by switching on and off at will the nano-valve represented by an incorporated MscL channel protein." | |
| Dudia is first author of a recent paper in Nano Letters that describes, for the first time, a novel polymer?lipid hybrid nanocontainer with controlled permeability functionality ("Biofunctionalized Lipid-Polymer Hybrid Nanocontainers with Controlled Permeability"). The hybrid nanodevice described in this paper was developed during the course of Dudia's PhD thesis "Nanofabricated biohybrid structures for controlled drug delivery" (pdf download, 14.2 MB) at the Biophysical Engineering Group and BMTI Institute for BioMedical Technology of the University of Twente. | |
| The "controlled permeability" concept has recently been developed by a multidisciplinary team at Biomade Technology Foundation and the University of Groningen, both in The Netherlands, and was subject of various papers: "A Light-Actuated Nanovalve Derived from a Channel Protein"in Science, "Rationally Designed Chemical Modulators Convert a Bacterial Channel Protein into a pH-Sensory Valve" in Angewandte Chemie International, and "Synthesis and utilization of reversible and irreversible light-activated nanovalves derived from the channel protein MscL" in Nature Protocols. | |
| "Due to the nature of the engineered channel protein, this functionality could be applied so far only in lipid bilayer systems, which somewhat limits the potentials of the system due to the stability of the bilayers" Dr. Armağan Koçer, one of the paper's co-authors, says. | |
| "By combining the strengths of my group on chemical modification of the functionality of proteins and those of Dr. Hans Kanger and Dr. Vinod Subramaniam on biophysical engineering, we managed to generate a hybrid system that can combine the desired properties of both polymers and lipid bilayers" says Koçer. | |
| Subramaniam, who holds the chair of Biophysical Engineering at the University of Twente, adds "Here we have used advanced focused ion beam nanofabrication techniques to drill nanometer scale holes into the impermeable polymer scaffolds. We then seal these holes with the functional lipid bilayers containing remote-controlled pore-forming channel proteins. This system allows exchange of solutions only after channel activation at will to form temporary pores in the container, which could not be managed before." | |
 |
|
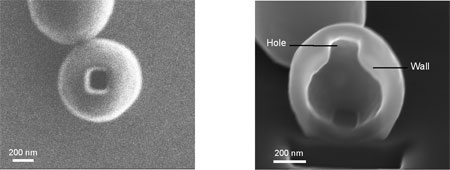
| Left: FIB image of a dried scaffold (outer diameter of 0.83 µm, wall thickness of 100 nm) containing a single hole of 200 nm x 200 nm. The hole was etched using a current of 1.5 pA. Right: FIB image of the scaffold with a hole rotated under an angle, where half of the scaffold was etched away using a current of 9 pA (Image: Dr. Dudia) | |
| Dudia explains that, by combining the advantages of both lipid and polymer containers, the researchers have generated, for the first time, an advanced biohybrid nanocontainer. "It is composed of a polymer vesicle with a lipid membrane patch covering a nanohole fabricated in the vesicle wall" she says. "The lipid membrane patch itself contains an engineered channel protein designed to allow for the passage of molecules up to a diameter of 3 nm. This system offers the controlled communication between the interior and exterior of the vesicle containers." | |
| "This is the first step towards the possibility of making a biomimetic equivalent of a cell – this development is the equivalent of the plasma membrane of a cell containing transmembrane proteins" she adds. "Of course, the cell plasma membrane is a hugely complex system, but this development captures the essence." | |
| As containers, liposomes suffer from limited stability and are leaky structures, whereas polymersomes are stable but do not allow instant release. In this novel hybrid system, polymer scaffolds form a robust architecture while a patch of sealed membrane serves as a natural environment for embedding pore-forming channel proteins and provides enough flexibility for the conformational changes that the channel requires to open a pore and release the payload. | |
| "The exciting feature of this hybrid system as a triggered delivery device is that these pore-forming channels can be engineered in order to respond to the unique triggers necessary for release at the target delivery site" says Koçer. | |
| Large-scale fabrication of these addressable hybrid nanocontainer still poses a problem. Currently they are made in a serial fashion but bulk fabrication methods need to be developed to make them useful for practical applications. Such potential applications could be found in a broad variety of research areas, ranging from fundamental single molecule biophysics and biochemistry to targeted drug delivery and molecular imaging. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
