| Posted: May 24, 2006 | |
Using nanofibers to repair the heart |
|
| (Nanowerk Spotlight) The human heart does not have significant regeneration capabilities and cardiologists look to cell therapy as a promising new method for cardiac repair. Now there is a new delivery system that improves the results of cell therapy. The new system allows greater control of the intramyocardial environment (inside the heart muscle) by delivering growth factors to an injured heart muscle and using peptide nanofibers for prolonged delivery of the injected factor. | |
| Dr. Richard T. Lee, a cardiologist at Harvard Medical School's Brigham and Women's Hospital (BWH), explained their recent research to Nanowerk: "One of the big problems with cell therapy is that once you inject cells into the body, you lose control over their environment. In the laboratory, we have tight control of conditions and can add growth factors, for example, whenever we want. The main finding of our study is that you can delivery growth factors to the environment around injected cells using nanofibers." | |
| Dr. Lee's recent paper, titled "Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction" was published in the May 12, 2006 online edition of Proceedings of the National Academy of Sciences (PNAS). The paper was first authored by Michael E. Davis and co-authored by collaborators from BWH and the Division of Biological Engineering at MIT. | |
| "Most past studies inject proteins directly into tissues like the heart" Lee says. "Many proteins delivered this way don't stay in the tissue very long. Our new technology allows the growth factor to be delivered for many weeks in a highly controlled way." | |
| Repairing the heart by using cell therapy is a very promising new approach to cardiac repair. However, studies to date indicate that few transplanted cells engraft and ultimately function normally within the host tissue. The shortcoming of many therapeutic strategies is the lack of quantitative control of transplanted cells in their new environment. | |
| Substantial data suggest that insulin-like growth factor 1 (IGF-1) is a potent cardiomyocyte growth and survival factor. IGF-1 overexpression increases cardiac stem cell number and growth, leading to an increase in myocyte turnover and function in the aging heart. However, IGF-1 is a small protein that diffuses readily through tissues, which restricts its retention within these tissues for prolonged periods. | |
| Self-assembling peptides are oligopeptides (polypeptides less than 30-50 amino acids long) composed of alternating hydrophilic and hydrophobic amino acids. On exposure to physiological osmolarity and pH, the peptides rapidly assemble into small nanofibers (10 nm) that can be injected into the heart muscle to form 3D cellular microenvironments. These peptide nanofiber microenvironments recruit a variety of cells including vascular cells. | |
| Lee explains: "In our article, we describe development of a delivery system using a "biotin sandwich" approach that allows coupling of a growth factor to peptide nanofibers without interfering with self-assembly. Biotinylation of self-assembling peptides allowed specific and highly controlled delivery of IGF-1 to local myocardial microenvironments, leading to improved results of cell therapy." | |
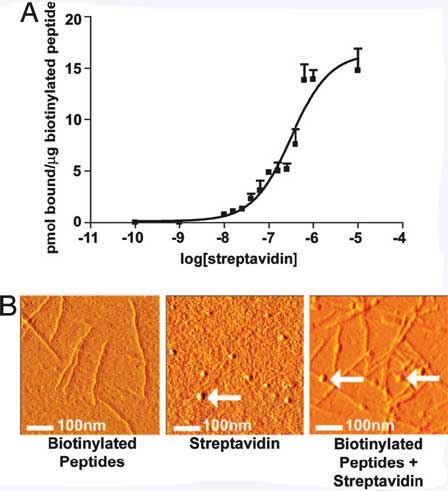 |
Streptavidin-binding to the biotinylated peptides. (A) Binding curve demonstrating the binding capacity of 35S-streptavidin to biotinylated selfassembling peptides. (B) AFM images of the biotinylated self-assembling peptide nanofibers alone (Left), streptavidin alone (Center), or streptavidin coupled to the biotinylated self-assembling peptide nanofibers (Right). Arrows show spherical streptavidin molecules. (Reprinted with permission from PNAS) |
| "Although we describe controlled myocardial delivery here, this approach may be used to delivery one factor or even multiple factors to tissues for prolonged periods" Lee says. | |
| This ability to control the local myocardial microenvironment may prove critical to preventing heart failure. | |
| Lee concludes: "We think this is just the beginning of how we can design the local environment around cells to encourage them to do things that can help the injured tissue. We just delivered one factor, but there's no reason you can't deliver many factors that are needed." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
