| Posted: Apr 07, 2008 | |
Using nanotechnology to improve Li-ion battery performance |
|
| (Nanowerk Spotlight) Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. | |
| In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. This not only applies to consumer electronics. As with so many other nanotechnology research, the military is a strong driver behind battery R&D. All of the electronic gizmos of a modern soldier - night vision goggles, flashlights, laptops, radios, GPS, etc. - are powered by batteries. The backup batteries soldiers are required to carry generally add several kilograms to their basic load, and the logistics necessary to supply the troops with sufficient numbers of replacement batteries is costly. | |
| To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. For instance, metallic tin has recently been widely researched as one of the promising anode materials for the next generation of high energy density lithium-ion batteries. This new anode material has a high theoretical specific capacity but its practical application is limited by its poor cycling performance. The problem with increasing the performance of lithium batteries is that none of the existing electrode materials alone can deliver all the required performance characteristics including high capacity, higher operating voltage, and long cycle life. Consequently, researchers are trying to optimize available electrode materials by designing new composite structures, often at the nanoscale. | |
| Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries. | |
| "Metallic tin is considered to be a very promising anode material for lithium batteries mainly for three reasons" Prof. Li-Jun Wan< explains to Nanowerk. "Firstly, its theoretical specific capacity is much higher than that of conventional graphite. Secondly, the tin anode has higher operating voltage than graphite, so it is less reactive and the safety of batteries during rapid charge/discharge cycle could be improved. And thirdly, a significant advantage of metallic tin over graphite is that it does not encounter solvent intercalation – which causes irreversible charge losses – at all. Unfortunately, the biggest challenge for employing metallic tin as applicable active anode materials is that it is suffering from huge volume variation during the lithium insertion/extraction cycle, which leads to pulverization of the electrode and very rapid capacity decay." | |
| Wan, Director of the Institute of Chemistry at the Chinese Academy of Sciences (CAS) in Beijing, PR China, together with members of the Beijing National Laboratory for Molecular Sciences, has published a paper in the February 27, 2008 online edition of Advanced Materials that describes this novel carbon nanocomposite as a promising anode material for high-performance lithium-ion batteries ("Tin-Nanoparticles Encapsulated in Elastic Hollow Carbon Spheres for High-Performance Anode Material in Lithium-Ion Batteries"). | |
 |
|
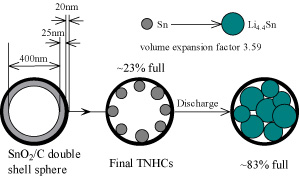
| A schematic illustration of the structure and the lithiation process of the tin nanoparticles encapsulated with elastic hollow carbon spheres. (Image: Dr. Wan) | |
| "Not only is this a further example of the directed nanostructure-design of electrode materials for lithium-ion batteries, but also the strategy could be extended to other anode and cathode materials by using elastic hollow carbon spheres as buffer and container" says Wan. | |
| The CAS scientists successfully have realized a novel structure design of tin-based anode material to solve the problem of capacity decay. The key to their composite material are nano-sized tin particles that are put into hollow carbon containers, resulting in tin nanoparticles encapsulated with elastic hollow carbon spheres (TNHCs). | |
| The TNHCs were prepared by in-situ reducing tin oxide hollow spheres with carbon coating. As described in their paper, the tin oxide spheres were firstly synthesized according the Stöber method and used as templates to prepare hollow spheres. Next, polycrystalline tin oxide was deposited on silicon dioxide spheres to form uniform shells. Then their cores were etched to get hollow spheres. After that, the carbon precursor layers were coated on the outer surface of the spheres. Finally the product was dried and heat-treated to carbonize the carbon precursor shell, and meanwhile the inner tin oxide shells were reduced to metallic tin by the carbon shells themselves to get the final TNHCs. | |
 |
|
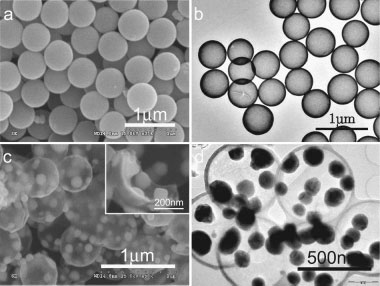
| a) SEM image of tin oxide coated silicon dioxide spheres; b) TEM image of the hollow tin oxide spheres; c) and d) SEM and TEM image of TNHCs, respectively. The inset in (c) is a close view of a single broken carbon spherical shell studded with tin particles. (Reprinted with permission from Wiley) | |
| These TNHCs with uniform size of ca. 500 nm diameter encapsulate multiple tin nanoparticles with a diameter of less than 100 nm in one thin hollow carbon sphere with a thickness of only about 20 nm. This nanocomposite material is characterized by a tin content of up to 74% by weight (which results in a high theoretical specific capacity of 831 milli-ampere hours per gram) and a void volume in the carbon shell as high as about 70?80% by volume. | |
| Wan explains that the approx. 1:3 tin nanoparticles to void volume ratio and the elasticity of the thin carbon spherical shell efficiently accommodate the volume change of tin nanoparticles due to the lithium-tin alloying-dealloying reactions, and thus prevent the pulverization of the electrode. "As a result, this type of tin-based nanocomposite has very high specific capacity of >800 milli-ampere hours per gram in the initial 10 cycles, and >550 milli-ampere hours per gram after the 100th cycle, as well as excellent cycling performance, exhibiting a great potential as anode materials in lithium-ion batteries." | |
| The researchers point out that their results successfully demonstrate the power of the strategy of using elastic hollow carbon spheres as buffer and container and could be extended to other anode and cathode materials. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
