| Posted: Apr 29, 2008 | |
Converting conventional nanotubes into superior carbon for batteries |
|
| (Nanowerk Spotlight) The race is on to develop the next generation of nanotechnology-enabled electrochemical energy storage devices, also knows as batteries. Lithium of course has long been recognized as an ideal material for energy storage due to its light weight and high electrochemical energy potential, as witnessed by the ubiquitous use of Li-ion batteries. There still seems to be considerable potential to further improve the performance characteristics of these Li-ion batteries (see our recent Spotlight: Using nanotechnology to improve Li-ion battery performance and this previous article: Nanotechnology batteries - the end of exploding batteries?). There have been many design approaches to creating lithium ion batteries but they usually share common features: The positive electrode is typically a lithium metal oxide, with various metals used such as cobalt, nickel, and manganese. The negative electrode is typically a carbon compound or natural or synthetic graphite. Researchers in Germany have now demonstrated a simple route for transforming cheap commercial carbon nanotubes into highly efficient carbon for electrochemical energy storage applications. When tested as electrode materials for lithium batteries, this composite material exhibits excellent performance over long test cycles. | |
| "We were able to demonstrate, for the first time, the template-free synthesis of carbon nanotube (CNT) encapsulated carbon nanofibers (CNFs@CNTs), where cheap and low-quality commercial carbon nanotubes are transformed into high-performance electrode materials" Dr. Dangsheng Su tells Nanowerk. "Compared to single-walled CNTs, the CNTs used in our study had a lower surface area, bigger outer diameter (50–200 nm), and thicker walls (50–100 walls). Large-scale production reduced their price to as little as $50 per kilogram." | |
| Su, who heads the Electron Microscopy & Microstructure Group in the Department of Inorganic Chemistry at the Fritz Haber Institute of the Max Planck Society in Berlin, Germany, together with colleagues from his institute and the Max Planck Institute for Solid State Research in Stuttgart, has found that this new class of carbon materials exhibits unique structural properties; which give it significant potential for applications in the field of gas adsorption, environmental protection, fuel cells, catalysis, hydrogen storage, etc. | |
| These results have been reported in the March 28, 2008 online edition of Advanced Materials ("CNFs@CNTs: Superior Carbon for Electrochemical Energy Storage") | |
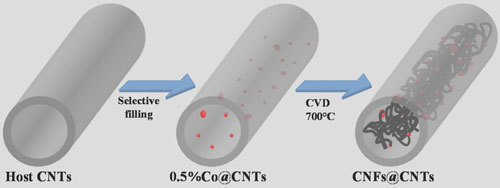
| Su explains that the CNFs@CNTs were synthesized via selective deposition of an active metal on the inner walls of CNTs, which was followed by the growth of carbon nanofibers (CNFs) by means of catalytic chemical vapor deposition (CCVD). | |
| "The Co@CNTs precursor, containing 0.5 wt% cobalt, was produced using a capillary force based incipient wetness impregnation methods, whereby a thin layer of cobalt nitrate solution is preferentially dispersed onto the inner surface of the CNTs. This causes the generation of metallic cobalt nanoparticles (average size: 6.6 nm) primarily on the inner wall of the CNTs during the subsequently performed H2 reduction step. These then act as active phase for CNF growth in the tubular chamber during the CCVD process." | |
 |
|
| Synthesis route to carbon-nanotube-encapsulated carbon nanofibers (CNFs@CNTs). (Reprinted with permission from Wiley-VCH Verlag) | |
| The researchers found that the CNFs@CNTs with a novel structure possessed a much better porosity than the pristine nanotubes. "Nitrogen physisorption tests showed that the specific surface area and the pore volume increased from 82 m2 per gram and 0.17 cm3 per gram to 347 m2 per gram and 0.61 cm3 per gram, respectively" says Dr. Jian Zhang from the Fritz Haber Institute. " The total increase in weight was 25% as measured after the CCVD process; which suggests a greatly improved utilization of space inside the hollow channels of the CNTs, thus an increase in their bulk density. This arises primarily from the formation of secondary pores between the CNFs and CNTs as well as the extremely close stacking of CNFs inside the CNTs, because the produced CNFs alone could not contribute to such a great extent." | |
| Su also notes that In terms of permanence of the high lithium storage capacity, CNFs@CNTs are superior to pristine CNTs. "During 120 cycles the reversible capacity of the CNFs@CNTs electrode stayed at around 410 mAh per gram while it gradually decreased to 258 mAh per gram when the electrode was formed from commercial CNTs." | |
| The researchers hypothesize that the superior stability of these CNFs@CNTs probably arises from a steric hindrance effect of their compact structure which suppresses the diffusion of big electrolyte molecules through wall defects. The confinement of CNTs suppresses the exfoliation of CNFs during intercalation/de-intercalation of lithium ions, give the long time stability they observed. | |
| Su points out that their outstanding cycling performance in combination with their high storage capacity makes CNFs@CNTs much more attractive than other carbon materials previously reported in the literature, such as for instance multi-walled CNTs, hard carbon, and CNFs. | |
| "Our fabrication method can be extended to other carbon materials (mesoporous carbon, activated carbon, carbon nanocones, etc.) as well as one-dimensional inorganic nanotubes and nanofibers" he says. | |
| For now, the scientists are working on the challenge of increasing the graphitization degree of carbon nanofibers inside CNTs. Eventually this could lead to much better performing Li-ion batteries. Another intriguing question is if this novel carbon composite material is suitable for hydrogen storage and at what performance. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
