| Posted: Jun 23, 2008 | |
Nanomechanical holography can reveal nanoparticles inside intact cells |
|
| (Nanowerk Spotlight) Nanomaterial-based drug-delivery and nanotoxicology are two of the areas that require sophisticated methods and techniques for characterizing, testing and imaging nanoparticulate matter inside the body. Especially the potential risk factors of certain nanomaterials have become a heated topic of discussion recently. Most, if not all, toxicological studies on nanoparticles rely on current methods, practices and terminology as gained and applied in the analysis of micro- and ultrafine particles and mineral fibers. The development of novel imaging techniques that can visualize local populations of nanoparticles at nanometer resolution within the structures of cells – without destroying or damaging the cell – are therefore important. | |
| Researchers in the U.S. have now demonstrated that at ultrasonic frequencies, intracellular nanomaterial cause sufficient wave scattering that a probe outside the cell can respond to. This ultrasonic holography technique provides a non-invasive way of looking inside a cell. | |
| "The novelty of our findings lie in the fact that it provides an alternative way to study a cell under ambient conditions, that is, without placing it in a vacuum, coating it with a metal, bombarding it with electrons, or inserting other molecules etc as is the case with other techniques such as electron microscopy or florescent tagging" Dr. Ali Passian explains to Nanowerk. | |
| Passian, a researcher with the Nanoscale Science and Devices Group at the Oak Ridge National Laboratory (ORNL) in Tennessee, and a Professor of Physics at the University of Tennessee, together with collaborators from ORNL, the University of Tennessee and Northwestern University, has published the findings in the June 22, 2008 online edition of Nature Nanotechnology ("Imaging nanoparticles in cells by nanomechanical holography"). Co-authors of the paper are Laurene Tetard, Katherine Venmar, Rachel Lynch, Brynn Voy and Thomas Thundat from ORNL and Gajendra Shekhawat and Vinayak Dravid of Northwestern University. | |
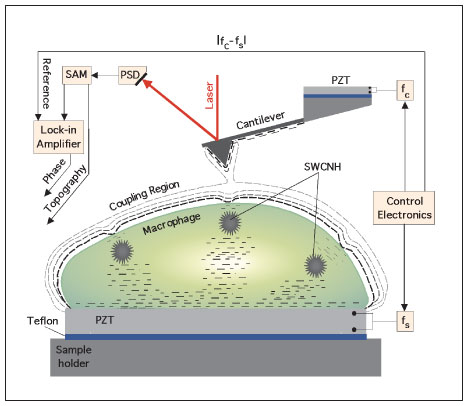 |
|
| Intracellular imaging of aspirated nanoparticles using ultrasonic holography. The signal access module (SAM) provides the instantaneous location of the reflected laser beam as monitored by a position-sensitive detector (PSD). The dynamics of the cantilever is presented at the input of a lock-in amplifier. The local perturbation in the coupled oscillations of the ultrasonic-driven micro cantilever - macrophage system is monitored with the lock-in using the difference frequency fc-fs as reference. By mapping the strength of the coupling in a scanned area of the cell, a phase image emerges that contains information on the buried SWCNHs. (Reprinted with permission from Nature Publishing Group) | |
| "The use of nanomaterial is becoming ubiquitous and as such a dire need exists to understand how engineered nanomaterial interact with biological species" Passian says. "Health effects and environmental factors are currently of major importance in our group at ORNL and a large number of our research resources have been focused on tackling the associated problems." | |
| He points out that with the novel imaging technique, scientists do not need to cut up the cell or inject artificial light-emitting molecules to find out whether or not a certain type of nanomaterial is present inside the cell or not. Therefore, one does not alter the intracellular configuration when attempting to pinpoint the nanoparticles. | |
| The recently developed technique known as Scanning Near Field Ultrasound holography (SNFUH) is a revolutionary approach which provides non-invasive nanoscale imaging capabilities for deeply buried and embedded structures. It offers the ability to acquire simultaneous topography and holography information with nanometer image resolution | |
| SNFUH synergistically integrates three disparate approaches: a unique combination of scanning probe microscope platform (which enjoys excellent lateral and vertical resolution) coupled to micro-scale ultrasound source and detection (which facilitates "looking" deeper into structures, section-by-section) and a novel holography approach (to enhance phase resolution and phase coupling in imaging). | |
| Applying these technique to biological structures, it offers a way to image soft samples and probe structures that are below their surfaces. For instance, if a cell is oscillated at megahertz frequencies using a piezoelectric crystal, the ultrasonic waves traveling through the oscillating cell structure may weakly drive an AFM cantilever that is in contact with the cell surface, as long as the elastic properties of the cell can support a propagation mode in the ultrasonic spectrum. | |
| In their work, Passian and his team explored the viability of SNFUH as a technique to probe cellular uptake of nanoparticles. Specifically, they tried to determine the cellular fate of single-walled carbon nanohorns (SWCNHs) using a mouse model to detect and visualize particles within lavage cells and blood. | |
| "We found that the nanoparticles cause sufficient phase change that can be measured with an external probe" he describes the research findings. "Bear in mind that the nanoparticles were not artificially placed within the cell but they got there as a result of exposing a living mouse to carbon nanohorns and then killing the mouse a few days later and preparing the sample cells." | |
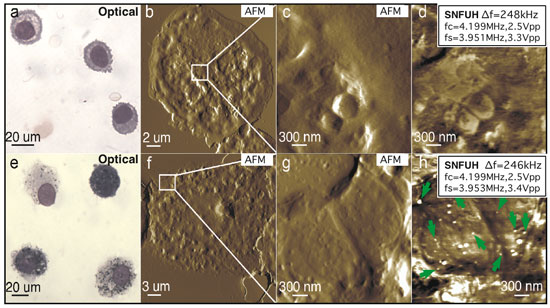 |
|
| Presence of SWCNHs inside cells obtained from mice lungs. a?h, Alveolar macrophages from vehicle control (a?d) and mice exposed to SWCNH after 7 days (e?h). Representative optical (a,e), AFM topography (b,c,f,g) and SNFUH (d,h) images from control and treated mice. White dots in h (some indicated by green arrows) correspond to SWCNHs. (Reprinted with permission from Nature Publishing Group) | |
| A striking visualization of the nanohorns was obtained from the SNFUH image of a macrophage collected from SWCNH-treated mice revealing several SWCNHs within the cell (marked with arrows in Fig. h above), which are not visible in the optical images Fig. d,g. | |
| "The sizes of these particles are statistically consistent with the size distribution (70?110 nm) established from analyzing several AFM images of the SWCNH solution, indicating that nanoparticles, rather than larger aggregates, were taken up by the macrophages" Passian explains. "The contrast measures the phase of the local tip?cell surface coupling, and originates from the difference in elasticity and density between the SWCNH and the cell." | |
| This work clearly demonstrates that specific problems that require subsurface knowledge can be tackled using Passian and his team's studies. This will clearly benefit research areas such as nanotoxicological investigations, drug delivery, pharmaceutical work, where, so far, most studies can only target the surface of samples and suffer from the lack of probes that can, with nanoscale resolution, provide information on what may be within a sample. | |
| Passian says that the team is pursuing to improve its capabilities so that a greater number of samples can be investigated. "In particular, a challenge we are targeting to overcome is the ability of using our approach to visualize the interior of a cell in their natural milieu, that is, in the case of a cell in a fluid." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
