| Posted: Jul 12, 2006 | |
Oscillating pattern in nanoparticle crystallization |
|
| (Nanowerk Spotlight) In order to survive, biological systems need to form patterns and organize themselves. Scientists at the Max Planck Institute for Colloids and Interfaces (MPI-KG) in Potsdam, Germany, have now combined self-organization with chemical pattern formation. They demonstrated that oscillating reaction patterns like that of a Belousov-Zhabotinsky reaction can not only be generated in a one-phase system like in all previous examples but also in a two-phase system like liquid-solid. | |
| Scientists are especially interested in oscillating chemical reactions. These occur when reaction products periodically and repeatedly change. Their behavior is of importance to many fields of study - including chaos research. That is because these reaction systems are always complex and far away from thermodynamic equilibrium. One particularly well-known example is the Belousov-Zhabotinsky reaction. In it, a colored indicator is used to make the reaction products of a coupled redox reaction visible. They typically take on the pattern of concentric circles, spreading out, for example, across a petri dish. | |
| Dr. Helmut Cölfen at the Colloid Department at the MPI-KGB explains the significance of their findings to Nanowerk: "Before, the oscillating Belousov-Zhabotinsky reaction was only known for chemical reactions in solution. We could now demonstrate that it can also take place in crystallization and self-organization processes." | |
| "On basis of these results, scientists can better explain chemical reactions which are out of thermodynamic balance, as well as biological pattern formation in nature" says Cölfen. "Furthermore, our results could lead to the creation of surfaces with new kinds of structures." | |
| The findings of the Max Planck team, titled "Formation of Self-Organized Dynamic Structure Patterns of Barium Carbonate Crystals in Polymer-Controlled Crystallization" were published in June 21, 2006 online issue of Angewandte Chemie International Edition. | |
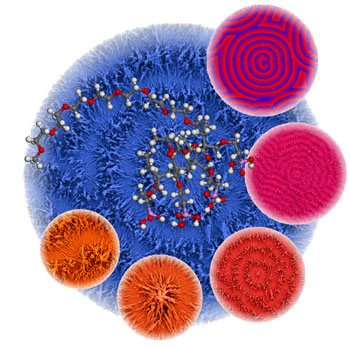 |
A typical Belousov-Zhabotinsky pattern of concentric circles, observed in this case in polymer-controlled crystallization and self-organization from barium carbonate. The structures are similar to a computer-simulated pattern (smaller circle, upper right). The block copolymer used appears in the picture as a shortened molecule structure. (Source: Max Planck Institute of Colloids and Interfaces) |
| The researchers used a newly synthesized polymer to create the typical concentric circle pattern, via controlled barium carbonate crystallization "Such patterns correspond quite well to the calculations in a simulation" says Cölfen. "We also were able to formulate a complex coupled reaction system including crystallization, complexation, and precipitation reactions and identify the autocatalytic formation of a complex between barium and the polymer." | |
| Notably, the elongated crystalline structures which made up the circular pattern are themselves created by superstructures of nanoparticles, which are themselves created by self-organization. | |
| Cölfen points out that such reactions could help with the understanding of pattern formation - also of natural patterns like those on mussel shells. In addition, reactions far away from thermodynamic equilibrium can be investigated. | |
| Looking ahead, Cölfen sees further research in expanding the mechanistic understanding of crystallization reactions far away from thermodynamic equilibrium and control of particle self-organization. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
