| Posted: Sep 24, 2008 | |
Nanotechnology produces highly effective radio frequency absorbers for cancer therapy |
|
| (Nanowerk Spotlight) Radio frequency ablation (RF ablation) is a treatment for cancer that works by inserting a thin needle guided by computed tomography or ultrasound through the skin and into a tumor. Electrical energy is then delivered through a number of electrodes deployed through the needle, causing a zone of thermal destruction that encompasses the tumor. | |
| Although minimally invasive, these procedures require the surgeon to insert the RF probes into the tumor and typically create a zone of non-specific tissue destruction 3-5 centimeters in size, with both malignant and normal tissues around the needle electrode undergoing thermal injury. Non-complete tumor destruction occurs in 5 to 40 percent of cases and complications arise in 10 percent of patients who suffer such damage. Radio frequency ablation is limited to liver, kidney, breast, lung and bone cancers. | |
| Researchers have experimented with non invasive radiowave thermal ablation of cancer cells that uses nanoparticles as a novel approach to treat cancer. The idea is that RF treatment of malignant tumors at any site in the body should be possible if it were possible to get agents that release heat under the influence of the RF field to the specific tumor site. Most RF ablation research so far has worked with gold nanoparticles – they are easily prepared and binding of cancer-targeting agents to the nanoparticles is easily achieved – though successful results have been demonstrated even with carbon nanotubes (see: Radio waves fire up nanotubes embedded in tumors, destroying liver cancer). | |
| Researchers have now developed a novel nanomaterial that has proven to be a very strong RF absorber and provides high enough thermal-ablation ability in order to generate localized thermally-driven damages inside the cancer cells. Even more, these nanoparticles have shown relatively low cytotoxicity and they absorb low frequency RF radiation, which has significant penetration depth inside living organism. Although several papers presented similar concepts, the nanomaterials used in this study proved to be superior at very low RF frequencies. | |
| "Our technique uses magnetic nanoparticles that are covered with graphitic layers as RF absorbers and localized heat providers" Dr. Alexandru S. Biris tells Nanowerk. "Moreover, we tried to understand the mechanisms that are responsible for the death of the cancer cells, such as DNA fragmentation, protein denaturation, development of holes in various membranes, etc. This work was motivated by the desire to find a method that can provide enough thermal energy into a tumor and to destroy it with RF energy provided from the outside of the body." | |
| In their work, Biris and his collaborators have demonstrated that graphitic carbon-coated ferromagnetic cobalt nanoparticles (C–Co-NPs) were efficiently uptaken by cervical cancer HeLa cells and acted as high efficiency radio frequency absorbers. After a short exposure time of between 2 to 30 minutes at low frequency (350 kHz) of RF radiation, the nanoparticles were found to be responsible for inducing cell death in over 98% of the cancer cells. The scientists believe that the RF-driven heating of the nanoparticles is responsible for the generation of extremely localized damages inside the cells. | |
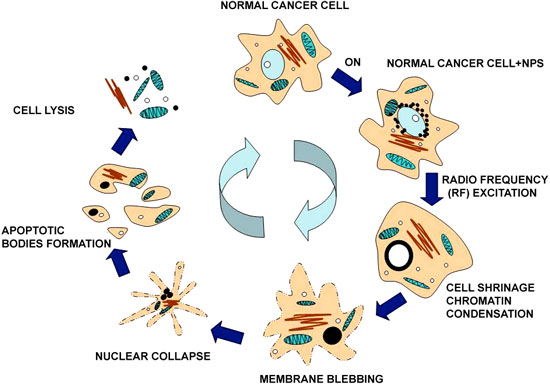 |
|
| A schematic diagram of HeLa cell with C–Co-NPs apoptosis process under RF excitation. (Reprinted with permission from IOP Publishing) | |
| Interestingly, most of the thermally induced biological modifications were found to happen inside the cellular sub-compartments. Biris, Chief Scientist and Assistant Professor at the University of Arkansas Nanotechnology Center, says that these results indicate that the cancer cells do not die because of their overall temperature increase, but rather due to the internal localized damages produced by the nanostructures. The research team also included scientists from the University of Arkansas for Medical Sciences, Electrostatics and Surface Physics Laboratory at NASA, the National Institute for Research and Development of Isotopic and Molecular Technologies in Romania, and from the AgCenter at Louisiana State University. | |
| The researchers determined that these nanoparticles clearly have the ability to cross the various inter-cellular membranes and to reach the nucleus. They observed that, after the nanoparticles are uptaken into the cells' cytoplasm, they tend to agglomerate around the nuclear membrane and a small number crossed the nuclear membrane into the nucleus. | |
| "Due to the localized RF heating provided by the nanoparticles, we found that the cells were going through an accelerated apoptotic process and consequently cellular decomposition" says Biris. "We believe that the disintegration of nano-localized cell environments such as nucleus, nuclear membranes, and DNA to be the main effect of the RF heat inducement into the cobalt nanoparticles." | |
| To further prove the function of the ferromagnetic metal nanoparticles, Biris and his team used single-wall carbon nanotubes as a comparison. Under identical conditions, only 3.1–6.6% of the cells were found to be dead versus 75.2–90.1% with cobalt nanoparticles. | |
| The results of this work, which was published in the September 22, 2008 online edition of Nanotechnology ("Cobalt nanoparticles coated with graphitic shells as localized radio frequency absorbers for cancer therapy"), indicate that such magnetic nanoparticles can become strong RF absorbers and therefore can generate thermally localized cellular damages which can induce cell death and cancerous tissue necrosis. | |
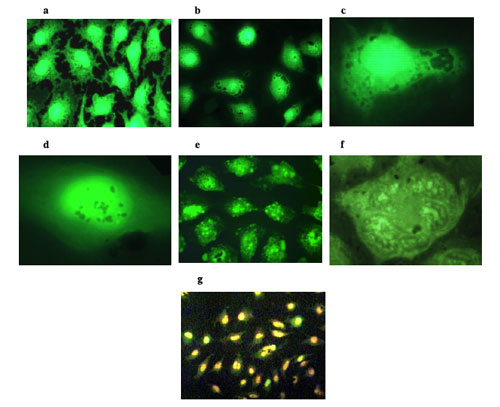 |
|
| Fluorescence microscopy images of HeLa cells. (a) Control HeLa cells. (b) After 24 hours incubation time, the C–Co-NPs were found to aggregate around and further penetrate into the nucleus of HeLa cells. (c) High magnification image of a HeLa cell nucleus surrounded by C–Co-NPs. (d) C–Co-NPs are being uptaken by the HeLa cells and they were found to cross and agglomerate inside the nucleus and cytoplasm. (e) Low magnification optical microscopy image indicating the nucleus fragmentation of HeLa cells incubated with C–Co-NPs and after a 350 kHz of RF heating for 20 min. (f) High magnification image indicating the nucleus fragmentation of HeLa cells incubated with C–Co-NPs and after a 350 kHz of RF heating for 20 min. (g) Low magnification optical image indicating the extensive death of the Co–NPs containing cells after they were exposed to the RF radiation for 20 min. The cells were stained in order to distinguish between the living (green for acridine orange) and the dead cells (orange for ethidium bromide). (Reprinted with permission from IOP Publishing) | |
| A main problem of non invasive radiowave thermal ablation is the requirement of providing enough thermal energy to the tumor site from the outside of the body. | |
| "We have demonstrated that using a combination of a low frequency, low power RF radiation – which has a high penetration ability in human tissue – with graphitic-magnetic composite nanoparticles could prove an excellent means of raising the temperature at the cellular level above the threshold required for DNA fragmentation or protein denaturation, which will ultimately be responsible for the death of the cells" Biris explains. | |
| The research team envisages several main directions developing around this particular research work: | |
| (1) use of such nanomaterials in vivo as high efficiency agents for the thermal ablation of cancer cells and tumors; | |
| (2) specific delivery of the carbon-magnetic nanomaterials to the tumors and their heating by single or combinations of electromagnetic sources (RF, lasers, X-Rays, magnetic, etc); | |
| (3) development of hybrid materials that can perform complex functions such as delivery of bio-pharmaceutical agents that can further enhance their thermal ablation properties, in order to accomplish an efficient killing of all the cancer cells; and | |
| (4) targeting , detection, and killing of single cancer cells in vivo in real time. | |
| According to Biris, the main problem that still needs to be addressed is the binding of their novel nanomaterial to antibodies, growth factors, amino-acids, etc for their efficient delivery to the tumor site. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
