| Posted: Nov 17, 2008 | |
Nanotechnology's complicated risk-benefit dichotomy |
|
| (Nanowerk Spotlight) Adding yet another twist to the emerging debate about the potential risks of nanomaterials, researchers have demonstrated how difficult it is to map out the health effects of nanoparticles. They have shown that, even if a certain nanoparticle does not appear toxic by itself, the interaction between this nanoparticle and other common compounds in the human body may cause serious problems to cell functions. On one hand, this effect could be used to great advantage in nanomedicine for killing cancer cells. On the other hand, unfortunately, it is unknown at present whether the same effect could be observed with healthy cells as well. Since the number of possible combinations of nanoparticles and various biomolecules is immense, it is practically impossible to research them systematically. This latest example of the risk-benefit dichotomy of nanotechnology just shows how thin the line is between promising applications such as effective cancer killers and the unknown risks posed by unintentional effects of exactly the same applications. | |
| "Our recent work with fullerenes provides two core findings," Dr. Emppu Salonen, a scientist at Helsinki University of Technology in Finland, tells Nanowerk. "Firstly, fullerenes, which are inherently insoluble in water, can be efficiently solubilized a phenolic acid which is ubiquitous in plants and can be found for instance in tea, grapes, oak bark, and cosmetics products as an antioxidant. Secondly, when exposed to gallic-acid-solubilized fullerenes, human tumor cells were shown to contract rapidly within tens of minutes and subsequently die." | |
| Salonen explains the significance of these two findings: | |
| "The first finding touches two important topics. First, there is intensive ongoing research for finding good solubilizing agents for carbon nanomaterials in general, in view of their use in nanotechnology applications. Second, while the volume of nanoparticles produced and the number of consumer products containing nanoparticles are both increasing rapidly, we still do not know much about their environmental and biological effects. We do not know, for example, how nanoparticles might get discharged into the environment, whether they could be solubilized in soil and natural waters, and wether they could enter the food chain. To us, that is quite many important unknowns." | |
| According to Salonen, the second finding has an interesting twist. Fullerenes are envisioned to be used in nanomedicine as drug delivery agents or even as the drugs themselves, for instance in therapies to fight cancer, HIV, Alzheimer's and Parkinson's diseases. | |
| "Fullerenes are excellent antioxidants and have already shown great promise in in vitro experiments. Gallic acid is abundant in for instance tea, red wines, walnuts and is used in cosmetics products due to its known antioxidant properties. What our work shows is that when acting together, these a priori beneficial compounds induce a fast and dramatic death of tumor cells. What we do not know at present, though, is whether the same effect could be observed also with healthy cells." | |
| Salonen is first author of a paper in the October 23, 2008 online edition of Small that describes these findings ("Real-Time Translocation of Fullerene Reveals Cell Contraction"). He worked with the research groups of Ilpo Vattulainen at Tampere University of Technology in Finland and Pu-Chun Ke at Clemson University in South Carolina. | |
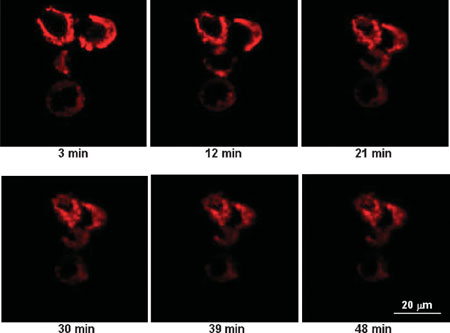 |
|
| Real-time interaction of C70-gallic acid and HT-29 cells. The cell membranes were labeled with lipophilic DiIC18 and their cross sections appeared as red 'rings'. Over time the cells were mechanically contracted due to the mutual interactions between C70-gallic acid nanoparticles (weight ratio 1:5). (Reprinted with permission from Wiley) | |
| The main motivation for this study was to understand the fate of fullerenes discharged into the environment since, with the increasing industrial-scale production and envisioned application of fullerenes in consumer products, their eventual entry into the environment is unavoidable. | |
| "The fact that nanoparticles are so small and highly reactive/adaptive makes determining their environmental impact much more challenging as compared to other, larger-size pollutants common today" says Salonen. "What we endorse – like many other researchers, governments and non-governmental organizations around the world – is proactive work on determining the biological and environmental effects of nanomaterials in general." | |
| The Finnish-U.S. research team integrated results from both atomistic molecular dynamics simulations conducted by Salonen and Vattulainen at their labs in Finland, and experimental observations by Ke's lab at Clemson. | |
| The toxicity of fullerenes is being intensively debated. As we have written in previous Nanowerk Spotlights, the often conflicting research reports with regard to the toxicity of certain nanoparticles have to do with the different ways these particles are synthesized or characterized. See for instance The challenge of designing nanomaterials with reduced toxicity with regard to fullerene functionalization, Comparing apples with oranges - the problem of nanotubes risk assessment with regard to carbon nanotubes, or a more general article on categorization frameworks (Nanotechnology risk assessment could benefit from nanoparticle categorization framework). | |
| The scientists used a naturally occurring phenolic acid – which is ubiquitous in fruits and all plant ecological system – as their solubilizing agent. They found that cells exposed to fullerenes or gallic acid separately did not show any loss of viability. However, it was the interplay of fullerenes and gallic acid combined that resulted in the dramatic cell death observed both as clear contraction of the cell plasma membranes in fluorescent measurements as well as directly in cell viability assays that provide information on the metabolism of cells. | |
| Basically, the study tried to answer two questions: 1) can fullerenes discharged into the environment be solubilized by some naturally abundant compounds (answer: yes), and 2) what are the possible biological effects of such fullerene-solubilizing agent aggregates (answer: apart from the observed contraction of cell membranes, mostly unknown). | |
| One area the scientists are already working on is studies to determine the effects of fullerene-gallic acid aggregates on cell plasma membranes, in order to provide an explanation for the cell death observed here. | |
| The particularly pressing question how fullerenes solubilized by gallic acid can enter the food chain and eventually interact with animal cells, remains unanswered, although Ke's group has already carried out experimental work on this topic and also with other carbon nanomaterials and with other solubilizing agents. | |
| Notwithstanding the potential negative effects, this research shows that gallic acid seems to be a very good compound for rendering fullerenes water soluble. | |
| Salonen says that it should be noted that the cell lines which were exposed to fullerene-gallic acid aggregates in this study were human tumor cells (HT-29 cell line). "But while it sounds very promising that fullerene-gallic acid aggregates were so effective in killing tumor cells, we still have no idea whether these nano aggregates could also induce similar effects with healthy animal cells and bacteria" he says. | |
| Salonen and his collaborators point out that the fact that gallic acid is such an efficient solubilizing agent of fullerenes raises an important question: Since gallic acid shares structural similarity with many components of natural organic matter (NOM) – a heterogeneous distribution of organic compounds found in soil and natural waters – it is valid to ask whether different chemical species in NOM can solubilize carbon nanomaterials as well. If so, what are the implications for environmental transport, bioavailability and physiological effects? | |
| "Understanding these effects in all of their complexity is a daunting task for future research" says Salonen. "Only by understanding the fate of nanomaterials in biological organisms and in the environment can we guarantee a safe and transparent development of nanotechnology." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
