| Posted: Nov 19, 2008 | |
Phase maps of nanotechnology materials aid in assessing their environmental impact |
|
| (Nanowerk Spotlight) Titanium dioxide nanoparticles have become a commercially significant nanomaterial and are being used in products around the world – in cosmetics and sunscreen lotions, paint formulations, coatings, self-cleaning additives, even in antibacterial applications. The increased use of nanomaterials such as titania goes hand in hand with a growing number of reports on the risks associated with these materials, which have arisen because insufficient information has been gathered about their reactivity and stability once they leave the laboratory. Unfortunately, pinpointing every conceivable situation that nanoparticles could interact in is an enormous multi-parameter problem and solving this by experimental testing alone is not feasible due to the huge numbers of combinatorial variations. | |
| According to Dr. Amanda Barnard, Future Generation Fellow at the School of Chemistry at the University of Melbourne, this is where theoretical predictions can help, by rapidly and systematically sampling possibilities, and highlighting where experimentalists should focus their attention. | |
| We have written about this issue of mathematical modeling in a previous Spotlight (Mathematical engines of nanomedicine) were we introduced the concept of design maps for the development of optimized nanoparticles for applications in nanomedicine. | |
| "The incorporation of more experimentally relevant parameters into theoretical descriptions of nanomaterials is important for our understanding of the stability of nanostructures in different chemical environments," Barnard explains to Nanowerk. "Using a size-, shape-, and temperature-dependent thermodynamic model we have generated the first phase map for anatase and rutile titania nanocrystals that includes both the equilibrium shape and the affects of surface chemistry." | |
| Barnard has worked with Dr. Huifang Xu, an Associate Professor Department of Geology and Geophysics at the University of Wisconsin, to develop a nanoscale phase diagram for titania in water. | |
| "This is the first complete nanoscale phase diagram for any oxide material, and the first – for any material – to include the effects of the surrounding solution," says Barnard. "It provides a predictive map of the anatase-rutile phase transition as a function of size and temperature, and accounts for all morphological transitions, that is, changes in particle shape, along the way. It is both proof-of-concept in terms of theoretical capability and the final piece in the nano-titania phase-stability puzzle." | |
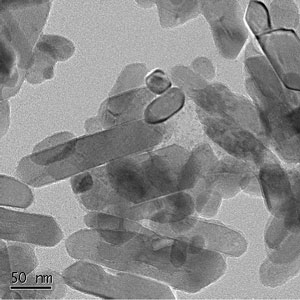  |
|
| Left: TEM image showing rutile nanocrystals with diameters of 20-40 nm. Right: Illustration highlighting that to study environmental stability, nanoparticles (such as anatase) must be assumed to be in contact with air and water, and will be at finite temperatures. (Images: Dr. Barnard and Dr. Xu) | |
| Apart from specific size effects, researchers have found – pretty much by trial and error – that the crystalline phase (anatase or rutile) and the shape of individual titania nanocrystals are also critical parameters in determining their effectiveness for certain applications, for instance in photocatalysis. | |
| Barnard mentions that recent studies have demonstrated remarkable control over the morphology of titania nanostructures. "However, this requires very specific synthesis conditions which are dissimilar to the environment in which these materials will ultimately be used and the greater natural environment with which they are likely to interact" she says. "Predicting the effect changing the chemical environment will have upon titania nanostructures is highly desirable, but the incorporation of more environmentally relevant parameters into theoretical descriptions of nanomaterials is not trivial." | |
| The phase map that Barnard and Xu developed brings together knowledge that has scattered in the literature and frames it in one neat package. The two scientists say that the predictions of their model are in excellent agreement with years of observations as reported in the literature and at the same time the phase map explains some ambiguities that have plagued researchers in the field for many years. | |
| "We now know why consensus on the size-dependence of the anatase-rutile phase transition has been so elusive, and why obtaining and retaining phase-pure specimens is so difficult" says Barnard. "The aim of this work was to 'map out' a solution to the problem, and we have finally done that". | |
| In general, a phase map is a two-dimensional graphical representation of chemical equilibrium, indicating stability of solid polymorphs at a given temperature, composition, and/or pressure. It becomes more complex with nanoscale materials where the phase maps need another axis representing the particle's size and the calculations are more complicated since the surface rather than bulk effects may dominate a particle's performance. | |
| Barnard points out that predictions that can be derived from these phase maps provide a guide as to the stability of nanomaterials under different conditions, and highlight how nanoparticles may respond to environmental changes. This will allow scientists and engineers to prescribe ideal operating conditions, to design ideal storage media to ensure long term stability, and plan for safe disposal methods when needed. | |
| "In the case of titania nanoparticles, our results also offer predictions of the phase stability in water, which becomes important when sunscreens containing titania nanoparticle wash off," she says. | |
| Based on this methodology, is it possible to obtain similar results for other oxides such as aluminum oxide, indium oxide, tin oxide, zirconium oxide or cerium oxide. | |
| "These nanomaterials are candidates for a variety of applications and this is the direction we intend to pursue in the future" Barnard explains the next steps for this particular research. "However, the calculation of this type of phase diagram is not trivial and experimental verification is even more challenging. The generation of sets of nanoscale phase maps corresponding to different chemical and natural environments is highly desirable, but we need more systematic characterization of real specimens to compare to. Ultimately a predictive map of this type is only useful if it can be shown to be reliable. While it was possible to verify the predictions in the case of titania, the same cannot currently be said for other oxide materials at the nanoscale." | |
| Barnard and Xu published their findings in the November 7, 2008 online edition of ACS Nano ("An Environmentally Sensitive Phase Map of Titania Nanocrystals"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
