| Posted: Jan 05, 2009 | |
Investigating potential nanotechnology risks at the bottom of the food chain |
|
| (Nanowerk Spotlight) The flurry of recent announcements regarding reports, international cooperations, and new research activities that deal with the potential risks of manufactured nanomaterials is a clear indication that the field of nanotoxicology is gaining momentum – and not too soon. While there still is no coherent international approach to determining if and what risks are posed by what kind of nanotechnology materials, individual research groups are picking certain areas of concern and forge ahead with – often highly specific – toxicology studies. | |
| A lack of standards and definitions makes these early investigations hard to compare and sometimes they even contradict each other, a situation that is especially confusing in risk assessments of carbon nanotubes. Some studies, though, present findings that, on the face of it, are especially worrying in their potential implications and deserve much more attention to be sorted out one way or another. A recent report on the toxicity of metal nanoparticles in soil is such an example. | |
| "Previous work has investigated the toxicity of metal oxide nanoparticles to plants, aquatic invertebrates, algae, bacteria and different cell lines," Baoshan Xing tells Nanowerk. "The effect of metal oxide nanoparticles on soil nematodes has scarcely been investigated. Soil is the medium that ultimately receives the released nanoparticles. Soil microorganisms and invertebrates play a key role in soil fertility, decomposition processes, nutrient and energy flows. Nematodes are the most abundant multicellular animals in soil and their function is irreplaceable in the soil-food web." | |
| Xing, a professor in the Department of Plant, Soil & Insect Sciences at the University of Massachusetts and his co-authors (Dr. Huanhua Wang and Dr. Robert L. Wick) have just published a paper in the December 9, 2008 online edition of Environmental Pollution that addresses if metal oxide nanoparticles are more toxic than their bulk counterparts to C. elegans, especially to their reproductive capability, and further explains that the observed toxicity was not simply due to dissolved metal ions ("Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans"). | |
 |
|
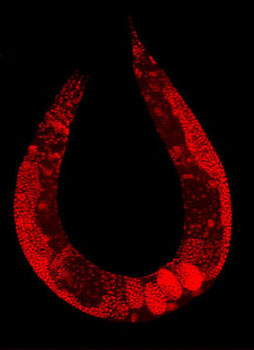
| Wild-type C. elegans hermaphrodite stained to highlight the nuclei of all cells (Image: Wikimedia Commons) | |
| The UMass team selected C. elegans because it is a widely used model organism in neurobiology, developmental biology and genetics due to its fast developmental biology, transparent body structure, complete genome sequence and unique biological features relevant to human disease. In addition, C. elegans or other nematodes could be attacked by predacious nematodes, insects and parasitic fungi, potentially transferring nanoparticles through the food chain where they could enter organisms higher up the chain. | |
| In natural ecosystems, nematode abundance and community structure analyses have proven to be sensitive indicators of stress caused by soil pollutants and ecological disturbance. Therefore, one may try to use the C. elegans to develop a sensitive indicator of nanoparticle toxicity; and to model nanoparticles uptake, accumulation, and transfer in organisms at higher trophic levels. | |
| Thus, this kind of study provides significant information about the potential environmental impact of nanoparticles. | |
| "In our work, both mortality and sublethal effects – growth, eggs inside worm body, and offspring per worm – of nanoparticles were determined," Xing explains. "We carried out parallel tests with bulk particles and dissolved metal ions, and also compared the toxicity between supernatant after centrifugation and filtration and nanoparticle suspension to clarify if the toxicity was caused by the particles per se or the dissolved metal ions." | |
| The team experimented with three types of commonly used metallic nanoparticles: zinc oxide, aluminum oxide, and titanium dioxide. They exposed C. elegans to both nanoparticulate and bulk versions of each metal in an aqueous exposure medium. The results show that these three metal oxides in nanoparticulate form are more toxic to C. elegans than in bulk form, especially to its reproductive capability. | |
| Xing points out that this toxicity could not be adequately explained by dissolution of the particles alone: "This conclusion is mainly based on two considerations: First, the lethal concentration values of aluminum oxide and titanium dioxide nanoparticles were significantly lower than their bulk particles, respectively; second, the toxicity of supernatant of the three tested metal nanoparticles after centrifugation and filtration at selected concentration was significantly lower than the corresponding suspensions of nanoparticles, especially for the number of eggs inside body and offspring per worm. Therefore, we think that these metal nanoparticles are more toxic than their bulk counterparts; and toxicity of the nanoparticles could not be explained by the dissolved ions alone, and a nanoparticle-specific toxic mechanism may exist." | |
| The researchers hypothesize that other possible toxicity mechanisms may include disruption of cell membranes, oxidation of proteins and enzymes, and formation of reactive oxygen species (ROS). "However" says Xing, "from our current research we do not know the exact location of nanoparticles in the C. elegans' body and active sites at which ROS production can take place." | |
| These findings suggest that follow-up studies should aim at identifying the mechanism of reproductive capability decrease of nematodes upon exposure to nanoparticles – an issue that could be critical to biodiversity and ecosystem health. | |
| Nematodes are pretty much at the bottom of the food chain and if they are capable of absorbing nanoparticles then another point of great interest and concern is the possible transfer and accumulation of nanoparticles through the food chain. | |
| Xing's experiments were conducted in a laboratory under controlled conditions. A major challenge for this and other toxicity studies is their application to the milieu and complex nature of real soils and their varying properties. | |
| "Natural organic matter in soil may interfere with biological systems via the induction of biotransformation enzymes, the production of internal oxidative stress, or through feminization," Xing explains. "Therefore, future research should investigate if natural organic matter affects nematode reproduction and has the potential to modulate the transcriptional response within the whole genome in the presence of metal oxide nanoparticles." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
