| Posted: Feb 05, 2009 | |
Nitrogen-doped carbon nanotube catalyst systems for low-cost fuel cells |
|
| (Nanowerk Spotlight) Platinum nanoparticles are widely used as the cathode material in hydrogen/oxygen fuel cells (like the polymer electrolyte fuel cell – PEFC) due to their efficiency in catalyzing the oxygen reduction reaction (ORR), the process that breaks the bonds of the oxygen molecules. Although platinum is still considered the state-of-the-art ORR catalyst, it does not exhibit good stability. Typically, catalyst performance degradation begins as soon as the catalyst is introduced into a fuel cell and continues until it is no longer active. | |
| Platinum can lose its effectiveness either by clumping together or by becoming 'poisoned' by carbon monoxide, requiring high hydrogen purity or higher catalyst densities for the fuel cell to stay effective. This, together with the high cost of platinum is seen as one of the major showstoppers to producing mass market fuel cells for commercial applications. | |
| Researchers have been searching for viable non-precious metal ORR catalysts – an effort that is by no means new but dates back to 1960s, and several different classes of non-precious metal catalysts have been investigated over the years (see for instance: "A new promising class of non-precious metal catalysts for fuel cells "). Recent intensive research efforts in reducing or replacing the platinum-based electrode in fuel cells have led to the development of new ORR electrocatalysts such as platinum-based alloys and nanowires (see: Nanotechnology is key to improving fuel cell performance), transition metal chalcogenides, carbon-nanotube-supported metal particles, enzymatic electrocatalytic systems, and even conducting poly(3,4-ethylenedioxythiophene) (PEDOT)-coated membranes. | |
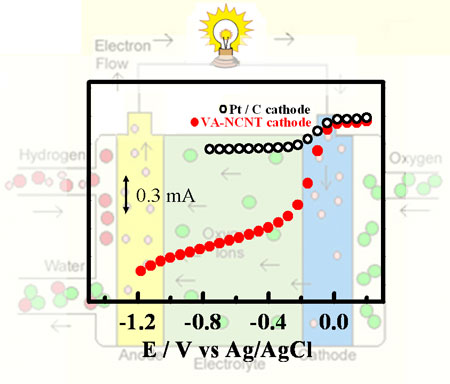
| Liming Dai, a professor of materials engineering at the University of Dayton (UD), and his group have been working on the growth and functionalization of aligned carbon nanotubes for some years. In a new study, they found that vertically-aligned nitrogen-containing carbon nanotubes (VA-NCNTs) produced by pyrolysis of iron(II) phthalocyanine (FePc, a metal heterocycle molecules containing nitrogen), in either the presence or absence of additional NH3 vapor, could be used as effective ORR electrocatalysts even after a complete removal of the residual iron catalyst by electrochemical purification. | |
| " We have shown that these metal-free VA-NCNTs catalyze a four-electron ORR process with a much higher electrocatalytic activity, more resistant to CO-poisoning, and better long-term operation stability even than that of commercially available or similar platinum-based electrodes in alkaline electrolytes" Dai explains to Nanowerk. | |
 |
|
| Nitrogen-doped carbon nanotube arrays can act as metal-free electrodes with a higher electrocatalytic activity than platinum for oxygen reduction in alkaline fuel cells. (Image: Dr. Dai, University of Dayton) | |
| Apart from these advantages, the high surface area, good electrical and mechanical properties, and superb thermal stability intrinsically characteristic of aligned carbon nanotubes provide additional benefits for the nanotube electrode to be used in fuel cells under both ambient and harsh conditions (e.g. for high temperature use). | |
| Dai says that the newly-found catalyst is thus better than platinum and its substitutes previously reported. | |
| The UD team, who worked with scientists from the Materials and Manufacturing Directorate of the Air Force Research Laboratory and the University of Akron, discovered that the incorporation of electron-accepting nitrogen atoms in the conjugated nanotube carbon plane makes adjacent carbon atoms carry a relatively high positive charge density. | |
| "This, coupled with the unique alignment structure, provides an efficient four-electron pathway for the oxygen reduction reaction on vertically-aligned nitrogen-doped carbon nanotubes," explains Dai."The process involves reduction of the positively-charged carbon atoms by the action of the electrochemical cycling and reoxidation of the reduced carbon atoms to their preferred oxidized state by reducing absorbed oxygen molecules." | |
| While these novel nitrogen-containing carbon nanotube electrodes are clearly of practical significance, the demonstration of the role of nitrogen-doping to create net positive charge on adjacent carbon atoms for facilitating the oxygen reduction reaction is an important finding in itself. It could be applied to the design and development of various other metal-free efficient ORR catalysts, even to new materials for applications beyond fuel cells. | |
| Dai and his team are already exploring novel low-cost nitrogen-containing catalyst systems for the oxygen reduction reaction while at the same time they continue work on their nitrogen-doped aligned carbon nanotube arrays to construct prototype fuel cell units. | |
| This work was published in the February 6, 2009 isue of Science (Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
