| Posted: Mar 11, 2009 | |
Metal nanoclusters have a bright future as luminescent probes for molecular sensors |
|
| (Nanowerk Spotlight) In physics, a plasmon is the quasi-particle resulting from the quantization of plasma oscillations just as photons and phonons are quantizations of light and sound waves, respectively. As the name indicates, surface plasmons are those plasmons that are confined to surfaces. The control of these surface plasmons has become increasingly attractive for optical signal processing, surface enhanced spectroscopy and sensor nanotechnology (also see our Spotlight: Plasmonics and optical tweezers – nanotechnology that manipulates with light). | |
| The plasmonic properties of nanoparticles depend on various parameters such as their size or shape, and the refractive index of the environment. Surface plasmons form the basis of localized surface plasmon resonance (LSPR) sensing, which allows the detection of single molecules. | |
| "In nanoclusters, the particle size is scaled down to approach the Fermi wavelength of an electron (ca. 0.5 nm for silver and gold), and the continuous density of states breaks up into discrete energy levels," Robin Ras explains to Nanowerk. "Therefore, optical and electronic properties of nanoclusters are significantly different to those of nanoparticles. However, the effect of environment on the properties of metal nanoclusters has not been reported to date." | |
| Ras and first author Isabel Díez, respectively senior researcher and postdoctoral researcher in the Molecular Materials group at Helsinki University of Technology, led by Olli Ikkala, are part of a Finnish-German research team that has demonstrated for the first time that the absorption and emission properties of few-atom metal nanoclusters respond dramatically to changes in the chemical environment, such as the molar composition and solvent. The team reports their findings in the February 10, 2009 online edition of Angewandte Chemie International Edition ("Color Tunability and Electrochemiluminescence of Silver Nanoclusters"). | |
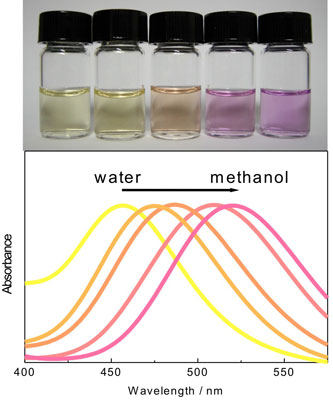
| Ras and his collaborators have systematically investigated the optical properties of silver (Ag) nanoclusters in solution – prepared in different water/methanol mixtures –and their response to the environment. What they found is that the spectral properties or color of the cluster solutions can be tuned to a great extent by selecting appropriate solvents. This property is called solvatochromism. The spectral shifts are not related to a change in nanocluster size. | |
 |
|
| Solvatochromic effect of silver nanoclusters. The figure illustrates the color shift in water-methanol mixtures under visible light. (Source: Dr. Ras, Helsinki University of Technology) | |
| "The clusters we examined were only few atoms in size such as Ag2 and Ag3" says Ras. "We found that the solvatochromism of the nanoclusters is analogous with that of metal nanoparticles but has a completely different origin. The solvatochromic effect for metal nanoparticles is well-known, and their photophysical properties are determined by surface plasmons. On the other hand, the photophysical properties of metal nanoclusters differ in character and are controlled by quantum confinement that results in discrete energy levels, therefore it was not evident that metal nanoclusters would also have solvatochromic properties." | |
| Researchers don't yet understand what the mechanism of the shift in color is. They hypothesize that perhaps it reflects a change in the electronic interaction between clusters, caused by a rearrangement of loosely bonded clusters over the polymer chains. | |
| Ras and his team, in collaboration with Dr. Y. Hancock (Helsinki University of Technology; Univ. York), are performing computational studies to unravel the mechanism behind the color shift. | |
| The solvatochromism of silver clusters could lead to new applications in sensing. Larger metal nanoparticles are already technologically important for LSPR sensing, which allows the detection of single molecules and finds applications in, for example, immunoassays and biochemical sensors. Whereas LSPR of nanoparticles is based on absorption of light, the silver clusters are in addition highly luminescent. | |
| It is well known that luminescence is much easier to detect than light absorption. Therefore metal clusters could lead to a significant increase in sensitivity compared to conventionally used nanoparticles. | |
| In addition, the Finnish-German team demonstrated that silver nanoclusters are electrochemiluminescent (i.e. electrogenerated chemiluminescence – ECL). | |
| ECL is a well-established technique in biomedical sensing. The low-cost instrumentation needed for ECL make it especially promising for point-of-care diagnostics, i.e. diagnostics to patients at their bedside. Silver nanoclusters have potential as luminescent labels for sensing by ECL. | |
| Ras points out that the synthesis method developed by his team is novel and produces metal nanoclusters without nanoparticle impurities. "In addition, the starting materials are cheap and commercially available, and the method is extremely facile and does not require chemistry skills" he says. "Basically all that is needed is to mix a silver salt with a polymer in water and to illuminate the solution under a conventional desk lamp." | |
| This means that metal clusters can become available in large quantities and at a low cost not only for chemists but also for scientists from other fields, like physicists and biologists. | |
| Having demonstrated that silver nanoclusters have potential as probes for sensing, the logical next step is to construct a sensor containing metal clusters as probe. | |
| "To prevent aggregation of the silver clusters to larger nanoparticles, or even to bulk silver, stabilizers are needed" says Ras. "Various compounds have been found suitable as stabilizer, including polymers, dendrimers, DNA, proteins and zeolites. However, little is known about the formation mechanism of silver clusters, or how the stabilizer interacts with the silver clusters. We need to develop a better understanding on this front, which in turn will certainly promote future research on clusters in general." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
