| Posted: Nov 01, 2006 | |
Fluorescent nanobodies could revolutionize biomedical research - thanks to camels |
|
| (Nanowerk Spotlight) Antibodies are large Y-shaped proteins used by the immune system to identify and neutralize foreign objects like bacteria and viruses. Each antibody recognizes a specific antigen unique to its target. That makes them valuable tools for the analysis of biomolecules in research, diagnostics and therapy. However, antibodies are huge (150 kDa) biomolecules and are not functional within a living cell due to the reductive environment of the cytoplasm. Normally, antibodies are used to detect antigens on fixed an permeabilized cells (in other words: dead cells). But neither does that provide any information about the dynamic changes of the antigen within different stages of the cell cycle, nor about its overall mobility. A research group at the University of Munich has now succeeded in developing much smaller molecules for antigen detection in living cells. | |
| Detection of particular antigens is a very common form of medical diagnostics and a very important tool in biomedical research. By combining the antibody for a specific biomolecular structure (e.g. a protein) with a marker, for instance a fluorescent dye, the presence of the structure within a cell or tissue can be demonstrated since the antibody will attach to its target. Unfortunately, this method doesn't work in living cells because the currently used conventional antibodies (produced mostly in mice, rabbits or goats) don't properly fold and assemble within living cells. | |
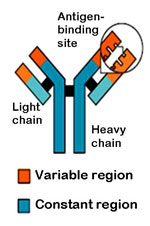 |
An antibody is made up of two heavy chains and two light chains. The variable region, which differs from one antibody to the next, allows an antibody to recognize its matching antigen. |
| Dr. Ulrich Rothbauer from the Leonhardt-Lab at the LMU Biozentrum (University of Munich/Germany) explained the novel molecules: "We fused the antigen-recognizing fragment of heavy-chain antibodies from camels and alpakas with fluorescent proteins to create fluorescent, antigen-binding nanobodies that can be expressed in living cells." | |
| The researchers call these molecular designs "chromobodies". These nanostructures combine the wide target range of antibodies with the live cell capabilities of fluorescent protein fusions. This means they potentially allow tracing of any cellular epitope, including their various conformational states. | |
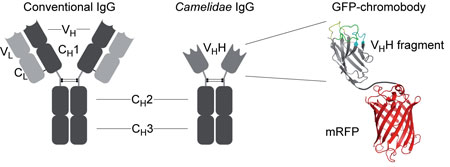 |
|
| Schematic outline of a conventional IgG antibody in comparison with a Camelidae-derived heavy-chain IgG antibody and a generic chromobody. The putative structure of the chromobody is shown at right, with the three antigen-binding loops in yellow, cyan and green, fused with a fluorescent dye (red). (Source: Leonhardt Lab) | |
| The coupling of antigen recognition and detection using a small molecule within a cell opens totally new ways e.g. for clinical diagnosis. This process isn't limited to proteins. It can be used to study their chemical modifications and other cell components as well. As a result, chromobodies can become valuable tools for target-screening, high cellular content analysis and especially in-cell imaging. | |
| "This approach could potentially revolutionize research in the areas of biomedicine, cell biology and proteomics" says Professor Heinrich Leonhardt. "Furthermore, this new technology offers an interesting and highly efficient alternative to conventional antibody production within animals. Thanks to chromobodies, the wide target range of antibodies can now be stored in artificial molecular databases." | |
| Rothbauer notes that the team already created databases with billions of chromobodies and successfully used this repository to create biotarget-specific probes. | |
| These findings are reported in a recent paper in Nature Methods ("Targeting and tracing antigens in live cells with fluorescent nanobodies"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
