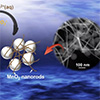
 New research shows how the photochemical reaction of nanoplastics through light absorption generates peroxyl and superoxide radicals on nanoplastic surfaces, and initiates oxidation of manganese into manganese oxide solids.
New research shows how the photochemical reaction of nanoplastics through light absorption generates peroxyl and superoxide radicals on nanoplastic surfaces, and initiates oxidation of manganese into manganese oxide solids.
Jan 7th, 2023
Read more
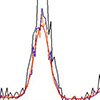 Sientists have successfully integrated analytical chemistry and informatics, using applied Bayesian inference to X-ray fluorescence analysis.
Sientists have successfully integrated analytical chemistry and informatics, using applied Bayesian inference to X-ray fluorescence analysis.
Jan 6th, 2023
Read more
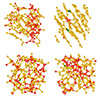 Offers the hope of converting coal to valuable and carbon-neutral materials key to electronic and battery technologies.
Offers the hope of converting coal to valuable and carbon-neutral materials key to electronic and battery technologies.
Jan 6th, 2023
Read more
 Although researchers have developed several methods to improve the reliability of nanotechnologies, the field still faces one major roadblock: a lack of a standardized way to analyze biological identity, or how the body will react to nanomedicines.
Although researchers have developed several methods to improve the reliability of nanotechnologies, the field still faces one major roadblock: a lack of a standardized way to analyze biological identity, or how the body will react to nanomedicines.
Jan 5th, 2023
Read more
 In a significant advance for impactful technologies such as quantum optics and laser displays for AR/VR, researchers have invented the first tunable and narrow linewidth chip-scale lasers for visible wavelengths shorter than red.
In a significant advance for impactful technologies such as quantum optics and laser displays for AR/VR, researchers have invented the first tunable and narrow linewidth chip-scale lasers for visible wavelengths shorter than red.
Jan 5th, 2023
Read more
 Researchers simulate the phase separation of self-spinning particles, and show that the process differs from other unmixing processes, which may shed light on the organization of bacteria and other organisms.
Researchers simulate the phase separation of self-spinning particles, and show that the process differs from other unmixing processes, which may shed light on the organization of bacteria and other organisms.
Jan 5th, 2023
Read more
 Adding charge acceptors can slow electron transfer in some light-activated materials.
Adding charge acceptors can slow electron transfer in some light-activated materials.
Jan 5th, 2023
Read more
 Researchers discover that electrons play a surprising role in heat transfer between layers of semiconductors, with implications for next-generation electronic devices.
Researchers discover that electrons play a surprising role in heat transfer between layers of semiconductors, with implications for next-generation electronic devices.
Jan 4th, 2023
Read more
 Although microbes are present on all organisms, the tools that estimate the safety of nanomaterials still hardly take them into account.
Although microbes are present on all organisms, the tools that estimate the safety of nanomaterials still hardly take them into account.
Jan 4th, 2023
Read more
 Perovskite solar cells degrade when exposed to sunlight, which results in decreasing performance over time. A new research project will examine how such solar cells could recover and self-repair at night.
Perovskite solar cells degrade when exposed to sunlight, which results in decreasing performance over time. A new research project will examine how such solar cells could recover and self-repair at night.
Jan 4th, 2023
Read more
 Scientists have overcome the scaling challenges of quantum optomechanical systems and realized the first superconducting circuit optomechanical graphene lattice.
Scientists have overcome the scaling challenges of quantum optomechanical systems and realized the first superconducting circuit optomechanical graphene lattice.
Jan 4th, 2023
Read more
 Chemical engineers have invented a solar-powered artificial leaf, built on a novel electrode which is transparent and porous, capable of harvesting water from the air for conversion into hydrogen fuel. The semiconductor-based technology is scalable and easy to prepare.
Chemical engineers have invented a solar-powered artificial leaf, built on a novel electrode which is transparent and porous, capable of harvesting water from the air for conversion into hydrogen fuel. The semiconductor-based technology is scalable and easy to prepare.
Jan 4th, 2023
Read more
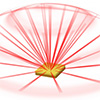 A novel laser cavity enables precise adjustment of the lasing direction over a wide range, with applications in LiDAR sensing.
A novel laser cavity enables precise adjustment of the lasing direction over a wide range, with applications in LiDAR sensing.
Jan 4th, 2023
Read more
 Researchers hope that ionocaloric cooling could someday help replace refrigerants with high global warming potential and provide safe, efficient cooling and heating for homes.
Researchers hope that ionocaloric cooling could someday help replace refrigerants with high global warming potential and provide safe, efficient cooling and heating for homes.
Jan 4th, 2023
Read more
 Unprecedented views of the interior of cells and other nanoscale structures are now possible thanks to innovations in expansion microscopy. The advancements could help provide future insight into neuroscience, pathology, and many other biological and medical fields.
Unprecedented views of the interior of cells and other nanoscale structures are now possible thanks to innovations in expansion microscopy. The advancements could help provide future insight into neuroscience, pathology, and many other biological and medical fields.
Jan 4th, 2023
Read more
 Researchers believe that emerging alternative semiconductors that are printable, low-cost and eco-friendly could lead the way to a cheaper and more sustainable IoT.
Researchers believe that emerging alternative semiconductors that are printable, low-cost and eco-friendly could lead the way to a cheaper and more sustainable IoT.
Jan 4th, 2023
Read more
 New research shows how the photochemical reaction of nanoplastics through light absorption generates peroxyl and superoxide radicals on nanoplastic surfaces, and initiates oxidation of manganese into manganese oxide solids.
New research shows how the photochemical reaction of nanoplastics through light absorption generates peroxyl and superoxide radicals on nanoplastic surfaces, and initiates oxidation of manganese into manganese oxide solids.















 Subscribe to our Nanotechnology News feed
Subscribe to our Nanotechnology News feed