Engineers tap DNA to create 'lifelike' machines
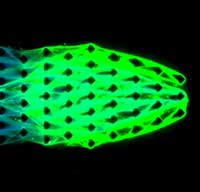 Tapping into the unique nature of DNA, engineers have created simple machines constructed of biomaterials with properties of living things.
Tapping into the unique nature of DNA, engineers have created simple machines constructed of biomaterials with properties of living things.
Apr 11th, 2019
Read more
 Tapping into the unique nature of DNA, engineers have created simple machines constructed of biomaterials with properties of living things.
Tapping into the unique nature of DNA, engineers have created simple machines constructed of biomaterials with properties of living things.
Apr 11th, 2019
Read more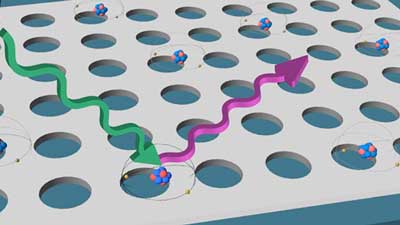 Patterned on a microchip and working in ambient conditions, the atoms could lead to rapid advancements in new quantum-based technology.
Patterned on a microchip and working in ambient conditions, the atoms could lead to rapid advancements in new quantum-based technology.
Apr 11th, 2019
Read more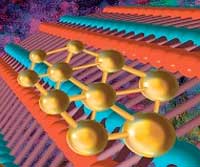 Two-dimensional (2D) semiconductors are promising for quantum computing and future electronics. Now, researchers can convert metallic gold into semiconductor and customize the material atom-by-atom on boron nitride nanotubes.
Two-dimensional (2D) semiconductors are promising for quantum computing and future electronics. Now, researchers can convert metallic gold into semiconductor and customize the material atom-by-atom on boron nitride nanotubes.
Apr 11th, 2019
Read more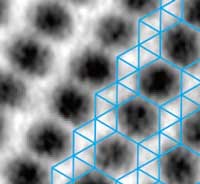 Researchers find new ways to image and characterize this unique material.
Researchers find new ways to image and characterize this unique material.
Apr 11th, 2019
Read more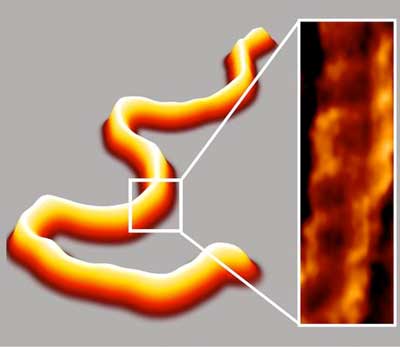 Scientists have developed a fast, simple sample preparation method that enhances imaging of DNA to better analyze its physical properties and interactions.
Scientists have developed a fast, simple sample preparation method that enhances imaging of DNA to better analyze its physical properties and interactions.
Apr 11th, 2019
Read more Physicists have developed a flexible way to synthesize a wide range of novel nanomaterials with potential applications in areas including optics and sensors.
Physicists have developed a flexible way to synthesize a wide range of novel nanomaterials with potential applications in areas including optics and sensors.
Apr 11th, 2019
Read more Researchers present the first hydrogen sensors ever to meet the future performance targets for use in hydrogen powered vehicles.
Researchers present the first hydrogen sensors ever to meet the future performance targets for use in hydrogen powered vehicles.
Apr 11th, 2019
Read more Scientists have developed a new microscopy approach for imaging gel nanocomposites in their natural state, which will reveal more useful information about their assembly and properties.
Scientists have developed a new microscopy approach for imaging gel nanocomposites in their natural state, which will reveal more useful information about their assembly and properties.
Apr 11th, 2019
Read more By speaking the brain's language, the material is a portal between electronics and the brain.
By speaking the brain's language, the material is a portal between electronics and the brain.
Apr 10th, 2019
Read more Tiny, individual, flexible ribbons of crystalline phosphorus have been made by researchers in a world first, and they could revolutionise electronics and fast-charging battery technology.
Tiny, individual, flexible ribbons of crystalline phosphorus have been made by researchers in a world first, and they could revolutionise electronics and fast-charging battery technology.
Apr 10th, 2019
Read more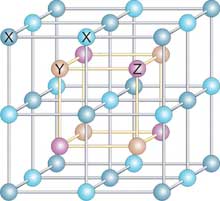 New quantum materials will one day make smartphones 1,000 times faster while drastically increasing battery life. That is how researchers describe the impact new semimetal materials could have on future electronics.
New quantum materials will one day make smartphones 1,000 times faster while drastically increasing battery life. That is how researchers describe the impact new semimetal materials could have on future electronics.
Apr 10th, 2019
Read more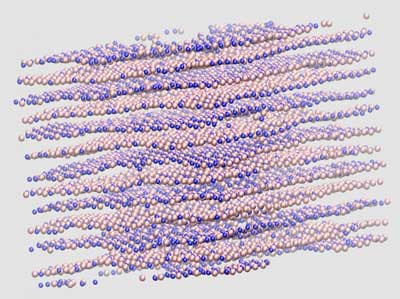 Engineers show faster techniques to model atom-flat materials for bottom-up design.
Engineers show faster techniques to model atom-flat materials for bottom-up design.
Apr 10th, 2019
Read more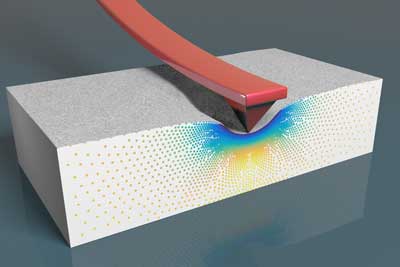 Scientists have theoretically and experimentally demonstrated that the piezoresponse force microscopy (PFM) technique can generate false positives when the piezoelectricity of a material is measured at the nanoscale.
Scientists have theoretically and experimentally demonstrated that the piezoresponse force microscopy (PFM) technique can generate false positives when the piezoelectricity of a material is measured at the nanoscale.
Apr 10th, 2019
Read more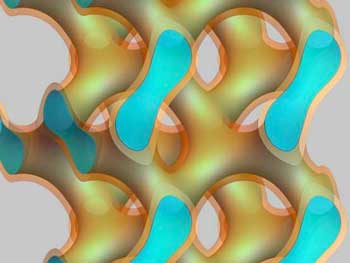 Can water reach minus 263 degrees Celsius without turning into ice? Yes it can, if it is confined in nanometre-scale lipid channels.
Can water reach minus 263 degrees Celsius without turning into ice? Yes it can, if it is confined in nanometre-scale lipid channels.
Apr 10th, 2019
Read more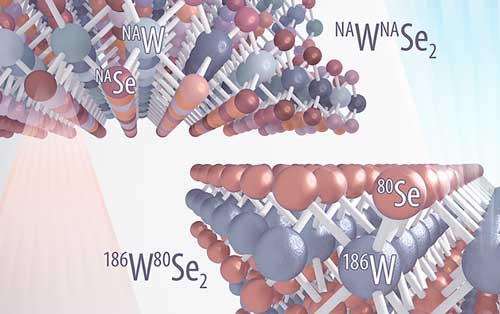 For the first time, researchers were able to grow an isotopically pure and highly uniform transition metal dichalcogenides (TMD) material only six atoms thick.
For the first time, researchers were able to grow an isotopically pure and highly uniform transition metal dichalcogenides (TMD) material only six atoms thick.
Apr 10th, 2019
Read more Researchers have synthesized new nanowires with high carrier mobility and fast infrared light (IR) response, which could help in high-speed communication.
Researchers have synthesized new nanowires with high carrier mobility and fast infrared light (IR) response, which could help in high-speed communication.
Apr 10th, 2019
Read more