Showing Spotlights 2385 - 2392 of 2779 in category All (newest first):
 Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry.
Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry.
Sep 19th, 2007
 There is a huge demand for medical implants for almost every body part you can think of. As we have reported here before, the market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, in many cases these metal alloys with a life span of 10-15 years may wear out within the lifetime of the patient. With recent advances in industrial synthesis of diamond and diamond-like carbon film bringing prices down significantly, researchers are increasingly experimenting with diamond coatings for medical implants. On the upside, the wear resistance of diamond is dramatically superior to titanium and stainless steel. On the downside, because it attracts coagulating proteins, its blood clotting response is slightly worse than these materials and the possibility has been raised that nanostructured surface features of diamond might abrade tissue. That's not something you necessarily want to have in your artificial knee or hip joints (although some of the currently used implant materials cause problems as well). Researchers have now run simulations that show that thin layers of ice could persist on specially treated diamond coatings at temperatures well above body temperature. The soft and hydrophilic ice multilayers might enable diamond-coated medical devices that reduce abrasion and are highly resistant to protein absorption.
There is a huge demand for medical implants for almost every body part you can think of. As we have reported here before, the market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, in many cases these metal alloys with a life span of 10-15 years may wear out within the lifetime of the patient. With recent advances in industrial synthesis of diamond and diamond-like carbon film bringing prices down significantly, researchers are increasingly experimenting with diamond coatings for medical implants. On the upside, the wear resistance of diamond is dramatically superior to titanium and stainless steel. On the downside, because it attracts coagulating proteins, its blood clotting response is slightly worse than these materials and the possibility has been raised that nanostructured surface features of diamond might abrade tissue. That's not something you necessarily want to have in your artificial knee or hip joints (although some of the currently used implant materials cause problems as well). Researchers have now run simulations that show that thin layers of ice could persist on specially treated diamond coatings at temperatures well above body temperature. The soft and hydrophilic ice multilayers might enable diamond-coated medical devices that reduce abrasion and are highly resistant to protein absorption.
Sep 18th, 2007
 Apart from buying a new computer every year it seems you need to upgrade your old machine on a regular basis to keep pace with ever bigger software packages and image files. Apart from the hassle of having to perform major surgery on your computer, these upgrades cost money. But, what if these upgrades were no longer necessary? What if your desktop computer came standard with the ability to store more data than you could ever possibly need and was able to function at unbelievable speeds? This would be too good to be true, right? Besides, who has the space for such a megacomputer. Well, imagine that this megacomputer could be packaged as a smaller device than current laptops, and cost only a fraction of today�??s prices? This sounds like hard core science fiction, but like so many radical science fiction ideas - the real thing might happen sooner than you think. As chip designers are nearing the physical limits of Moore's law (some say that the exponential increase in the cost of semiconductor production will most likely stop the current miniaturization trend before its physical limits are reached), scientists around the globe are working hard on developing the field of molecular electronics. An interdisciplinary science that includes physics, chemistry, nanotechnology, materials science and even biology, molecular electronics involves using molecular building blocks in the manufacture of electronic components. Driven by a growing interest in alternative concepts, like the integration of molecules as carriers of an electronic function, the electronics industry is poised to take the crucial step of integrating molecular devices into electronic circuits.
Apart from buying a new computer every year it seems you need to upgrade your old machine on a regular basis to keep pace with ever bigger software packages and image files. Apart from the hassle of having to perform major surgery on your computer, these upgrades cost money. But, what if these upgrades were no longer necessary? What if your desktop computer came standard with the ability to store more data than you could ever possibly need and was able to function at unbelievable speeds? This would be too good to be true, right? Besides, who has the space for such a megacomputer. Well, imagine that this megacomputer could be packaged as a smaller device than current laptops, and cost only a fraction of today�??s prices? This sounds like hard core science fiction, but like so many radical science fiction ideas - the real thing might happen sooner than you think. As chip designers are nearing the physical limits of Moore's law (some say that the exponential increase in the cost of semiconductor production will most likely stop the current miniaturization trend before its physical limits are reached), scientists around the globe are working hard on developing the field of molecular electronics. An interdisciplinary science that includes physics, chemistry, nanotechnology, materials science and even biology, molecular electronics involves using molecular building blocks in the manufacture of electronic components. Driven by a growing interest in alternative concepts, like the integration of molecules as carriers of an electronic function, the electronics industry is poised to take the crucial step of integrating molecular devices into electronic circuits.
Sep 17th, 2007
 Many of us don't like to admit it, but televisions are an important part of our lives. Technology has improved the quality and convenience of TVs and has given us a whole new set of choices - high definition, plasma, and liquid crystal displays. With an estimated 66 million sets to be sold this year, flat-screen LCD televisions are the most popular choice. Much of the reason behind the popularity of these high-tech wonders is the decrease in price that happens with most cool technology - a few years after they have been on the market. But, for the popular LCD TV, a shortage of a key compound used to produce LCDs may force manufacturers to raise prices. Electronics suppliers are facing a shortage of the rare metal indium, a co-product of zinc mining. Indium is a rare, malleable and easily fused metal, similar to aluminum, which is used to make indium tin oxide (ITO), the standard transparent electrode used in nearly all flat panel displays and microdisplays. Indium is expensive and scarce and demand is increasing. According to Displaybank, the demand for indium was 861 tons in 2006 and may reach nearly 2000 tons by 2011. Five years ago, indium was about $100/ kg; now it costs $800/kg. Displaybank expects the total sales of indium in 2007 to be $533 million. But, geologists say the cost of indium may not matter soon, because the earth's supply of it could be gone in four years. This could put a serious damper on that 52" LCD screen you've been dreaming about. Fortunately, with the help of nanotechnology, a team of scientists in Japan have developed a new material that may replace the need for indium in LCD production.
Many of us don't like to admit it, but televisions are an important part of our lives. Technology has improved the quality and convenience of TVs and has given us a whole new set of choices - high definition, plasma, and liquid crystal displays. With an estimated 66 million sets to be sold this year, flat-screen LCD televisions are the most popular choice. Much of the reason behind the popularity of these high-tech wonders is the decrease in price that happens with most cool technology - a few years after they have been on the market. But, for the popular LCD TV, a shortage of a key compound used to produce LCDs may force manufacturers to raise prices. Electronics suppliers are facing a shortage of the rare metal indium, a co-product of zinc mining. Indium is a rare, malleable and easily fused metal, similar to aluminum, which is used to make indium tin oxide (ITO), the standard transparent electrode used in nearly all flat panel displays and microdisplays. Indium is expensive and scarce and demand is increasing. According to Displaybank, the demand for indium was 861 tons in 2006 and may reach nearly 2000 tons by 2011. Five years ago, indium was about $100/ kg; now it costs $800/kg. Displaybank expects the total sales of indium in 2007 to be $533 million. But, geologists say the cost of indium may not matter soon, because the earth's supply of it could be gone in four years. This could put a serious damper on that 52" LCD screen you've been dreaming about. Fortunately, with the help of nanotechnology, a team of scientists in Japan have developed a new material that may replace the need for indium in LCD production.
Sep 14th, 2007
 New technology, whether it is a novel cancer treatment or an innovative approach to making a new material, almost always comes with risk. Nanotechnologies are no different. Certain nano-fabrication techniques employ toxic chemicals, the production of carbon nanotubes results in dangerous byproducts, and the big question as to what degree certain engineered nanoparticles could be harmful to humans and the environment has not been answered yet. The potentially adverse health effects of fine and ultrafine particles have been studied for decades. However, at the core of the nanotoxicological debate is the fact that nanoparticles are not just a smaller version of certain particles, but they are very different from their everyday counterparts with regard to their physical properties and catalytic activities. Thus their adverse effects cannot simply be derived from the known toxicity of the macro-sized material. One useful contribution to moving the nanotoxicology discussion further along came from the 1st Nobel Forum Mini-Symposium on Nanotoxicology that was held in Stockholm, Sweden. The event's program was devoted to the topic of definitions and standardization in nanotoxicological research, as well as nano-specific risk assessment and regulatory/legislative issues. A group of international experts presented examples of recent and ongoing studies of carbon-based nanomaterials, including single-walled carbon nanotubes, using a wide range of in vitro and in vivo model systems. This Spotlight will provide you with some highlights and conclusions from this exciting meeting.
New technology, whether it is a novel cancer treatment or an innovative approach to making a new material, almost always comes with risk. Nanotechnologies are no different. Certain nano-fabrication techniques employ toxic chemicals, the production of carbon nanotubes results in dangerous byproducts, and the big question as to what degree certain engineered nanoparticles could be harmful to humans and the environment has not been answered yet. The potentially adverse health effects of fine and ultrafine particles have been studied for decades. However, at the core of the nanotoxicological debate is the fact that nanoparticles are not just a smaller version of certain particles, but they are very different from their everyday counterparts with regard to their physical properties and catalytic activities. Thus their adverse effects cannot simply be derived from the known toxicity of the macro-sized material. One useful contribution to moving the nanotoxicology discussion further along came from the 1st Nobel Forum Mini-Symposium on Nanotoxicology that was held in Stockholm, Sweden. The event's program was devoted to the topic of definitions and standardization in nanotoxicological research, as well as nano-specific risk assessment and regulatory/legislative issues. A group of international experts presented examples of recent and ongoing studies of carbon-based nanomaterials, including single-walled carbon nanotubes, using a wide range of in vitro and in vivo model systems. This Spotlight will provide you with some highlights and conclusions from this exciting meeting.
Sep 13th, 2007
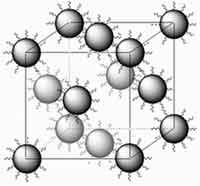 Zinc Oxide (ZnO) has long been used in its powdered form as pigments in paints, coatings for papers, in the commercial manufacture of rubber goods as well as UVA and UVB blocker and mild antimicrobial in cosmetics. ZnO is also one of the most important semiconductor compounds and numerous reports have been documented in the literature about the preparation and characterization of ZnO nanocrystals. While polycrystalline forms of ZnO have been used for technical uses such as piezoelectric transducers, light emitting diodes, and transparent conducting films, the progress in developing single crystal bulk ZnO have brought its promise as a wide band gap semiconductor to the fore. Superstructures formed from ZnO nanocrystal quantum dots may find applications in various areas such as optics, electronics and magnetism. For these 2D and 3D superstructures to be useful they need to be well-ordered. Usually, nanocrystals without any surface modification are less stable and they usually undergo aggregation or crystal growth, and consequently it is rather hard for bare nanocrystals to self-assemble into 2D, and especially into 3D, ordered structures. So far, most well-ordered assemblies of nanocrystals have been prepared through a surface modification approach. Efforts have been made to prepare superstructures composed of ZnO nanocrystals but it is rather challenging to obtain well-ordered 3D ZnO superlattices. Researchers in China have now found that ZnO nanocrystals capped with ionic liquids spontaneously assemble into a three-dimensional lattice. Apparently, simply drying a solution of the modified ZnO nanocrystals is all that is needed for the superlattice to form. The presence of the ionic liquid prevents the nanocrystals from aggregating.
Zinc Oxide (ZnO) has long been used in its powdered form as pigments in paints, coatings for papers, in the commercial manufacture of rubber goods as well as UVA and UVB blocker and mild antimicrobial in cosmetics. ZnO is also one of the most important semiconductor compounds and numerous reports have been documented in the literature about the preparation and characterization of ZnO nanocrystals. While polycrystalline forms of ZnO have been used for technical uses such as piezoelectric transducers, light emitting diodes, and transparent conducting films, the progress in developing single crystal bulk ZnO have brought its promise as a wide band gap semiconductor to the fore. Superstructures formed from ZnO nanocrystal quantum dots may find applications in various areas such as optics, electronics and magnetism. For these 2D and 3D superstructures to be useful they need to be well-ordered. Usually, nanocrystals without any surface modification are less stable and they usually undergo aggregation or crystal growth, and consequently it is rather hard for bare nanocrystals to self-assemble into 2D, and especially into 3D, ordered structures. So far, most well-ordered assemblies of nanocrystals have been prepared through a surface modification approach. Efforts have been made to prepare superstructures composed of ZnO nanocrystals but it is rather challenging to obtain well-ordered 3D ZnO superlattices. Researchers in China have now found that ZnO nanocrystals capped with ionic liquids spontaneously assemble into a three-dimensional lattice. Apparently, simply drying a solution of the modified ZnO nanocrystals is all that is needed for the superlattice to form. The presence of the ionic liquid prevents the nanocrystals from aggregating.
Sep 12th, 2007
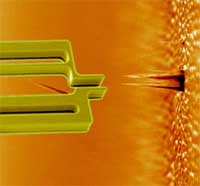 Hands-on nanotechnology: towards a nanorobotic assembly line (Nanowerk Spotlight) Until the twentieth century, a single craftsman or team of craftsmen would normally create each part of an industrial product individually and assemble them together into a single item, making changes in the parts so that they would fit together and work together; the so-called English System of manufacture. Then Henry Ford came along and in 1907-08 developed the assembly line for his Model T automobile. This innovation revolutionized not only industry but also our society because it allowed mass production of industrial goods at much lower cost than before. At its core, an assembly line is a manufacturing process in which interchangeable parts are added to a product in a sequential manner to create a finished product. Nanotechnology techniques today are pretty much where the industrial world was before Ford's assembly line - a domain of craftsmen and not of mass production. It has long been a dream for nanotechnologists that robots could one day be used in a similar way to produce nanodevices. A group of researchers from Denmark and Germany have now developed the rudimentary beginnings of the nanotechnology equivalent of an assembly line. They have shown 'pick-and-place' assembly of a working device using a silicon gripper - a robotic 'hand' some 10000 times smaller than a human hand. This nanogripper, controlled by a nanorobotic arm, is capable of picking up a carbon nanofiber (CN) and fix it onto the tip of an atomic force microscope cantilever.
Hands-on nanotechnology: towards a nanorobotic assembly line (Nanowerk Spotlight) Until the twentieth century, a single craftsman or team of craftsmen would normally create each part of an industrial product individually and assemble them together into a single item, making changes in the parts so that they would fit together and work together; the so-called English System of manufacture. Then Henry Ford came along and in 1907-08 developed the assembly line for his Model T automobile. This innovation revolutionized not only industry but also our society because it allowed mass production of industrial goods at much lower cost than before. At its core, an assembly line is a manufacturing process in which interchangeable parts are added to a product in a sequential manner to create a finished product. Nanotechnology techniques today are pretty much where the industrial world was before Ford's assembly line - a domain of craftsmen and not of mass production. It has long been a dream for nanotechnologists that robots could one day be used in a similar way to produce nanodevices. A group of researchers from Denmark and Germany have now developed the rudimentary beginnings of the nanotechnology equivalent of an assembly line. They have shown 'pick-and-place' assembly of a working device using a silicon gripper - a robotic 'hand' some 10000 times smaller than a human hand. This nanogripper, controlled by a nanorobotic arm, is capable of picking up a carbon nanofiber (CN) and fix it onto the tip of an atomic force microscope cantilever.
Sep 11th, 2007
 For the builders and engineers among you, our subject today is cement. Not necessarily a material one would associate with high-tech, not to mention nanotechnology. However, it's probably fair to say that our modern society is built on cement. Look around you and you'll find it everywhere - in buildings, roads, bridges, dams. Early construction cement (the word goes back to the Romans who used the term opus caementitium to describe masonry which resembled concrete and was made from crushed rock with burnt lime as binder) probably is as old as construction itself. So what is it? Cement, as it is commonly known, is a mixture of compounds made by burning limestone and clay together at very high temperatures. Cement is then used, together with water, as binder in a synthetic composite material known as concrete. For concrete to obtain its optimal properties it needs to harden. And that takes time. For builders, time is money and particularly in industrial settings time is a major cost issue. Time is also a safety and convenience factor, think about infrastructure repair work on roads and dams for instance. Cement manufacturers have already known that reducing the particle size of cements results in faster-binding formulations. By taking the ultimate reduction down to the nanoscale, researchers in Switzerland have shown that a one-step preparation of nanoparticulate cement with a conventional Portland cement composition results in a drastically increased early reactivity of the cement.
For the builders and engineers among you, our subject today is cement. Not necessarily a material one would associate with high-tech, not to mention nanotechnology. However, it's probably fair to say that our modern society is built on cement. Look around you and you'll find it everywhere - in buildings, roads, bridges, dams. Early construction cement (the word goes back to the Romans who used the term opus caementitium to describe masonry which resembled concrete and was made from crushed rock with burnt lime as binder) probably is as old as construction itself. So what is it? Cement, as it is commonly known, is a mixture of compounds made by burning limestone and clay together at very high temperatures. Cement is then used, together with water, as binder in a synthetic composite material known as concrete. For concrete to obtain its optimal properties it needs to harden. And that takes time. For builders, time is money and particularly in industrial settings time is a major cost issue. Time is also a safety and convenience factor, think about infrastructure repair work on roads and dams for instance. Cement manufacturers have already known that reducing the particle size of cements results in faster-binding formulations. By taking the ultimate reduction down to the nanoscale, researchers in Switzerland have shown that a one-step preparation of nanoparticulate cement with a conventional Portland cement composition results in a drastically increased early reactivity of the cement.
Sep 10th, 2007
 Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry.
Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





