Showing Spotlights 465 - 472 of 624 in category All (newest first):
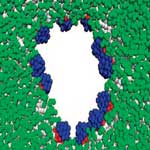 Nanotechnology researchers have appropriated the name of Janus - the Roman god of gates and doorways, usually depicted with two heads looking in opposite directions - to name a class of amphiphilic (i.e. containing both hydrophobic and hydrophilic portions) nanoparticles composed of two fused hemispheres, each made from a different substance. Their particular structure makes Janus particles an intriguing subject for exploring novel anti-cancer therapies where they, for instance, carry two different and complementary medicines. In a novel use of Janus particles, researchers have now isolated a means of using them to make 're-sealable' pores in lipid bilayer membranes. Described in another way, the localization of the nanoparticles in the pore can be thought of as the placement of a zipper, which allows a specific slit to be opened or closed at will.
Nanotechnology researchers have appropriated the name of Janus - the Roman god of gates and doorways, usually depicted with two heads looking in opposite directions - to name a class of amphiphilic (i.e. containing both hydrophobic and hydrophilic portions) nanoparticles composed of two fused hemispheres, each made from a different substance. Their particular structure makes Janus particles an intriguing subject for exploring novel anti-cancer therapies where they, for instance, carry two different and complementary medicines. In a novel use of Janus particles, researchers have now isolated a means of using them to make 're-sealable' pores in lipid bilayer membranes. Described in another way, the localization of the nanoparticles in the pore can be thought of as the placement of a zipper, which allows a specific slit to be opened or closed at will.
May 21st, 2008
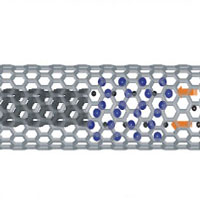 As far as test tubes go, it doesn't get any smaller than a single-walled carbon nanotube (SWCNT). Among the wide range of interesting properties exhibited by SWCNTs is their capacity to encapsulate molecules within their quasi one-dimensional cavity. The confinement offered by the nanotube could serve as a nanoscale test tube to constrain a chemical reaction. This was demonstrated in principle back in 1998, when the coalescence of adjacent fullerenes was observed by transmission electron microscopy. In the following years, scientists have extensively experimented with filling nanotubes with other fullerenes, atoms, molecules and, very recently, with organic molecules. Owing to their large variety with diverse chemical properties, the incorporated organic molecules can tune the properties of the SWCNTs. Scientists are intrigued by the possibilities that SWCNTs' use as a reaction tube offers for chemistry at the nanoscale. Nanochemistry - a key to control self-assembly processes prerequisite for nanotechnology - in essence would produce stable chemical reactions inside a confined nanoscale space. Encapsulated inside this nanoscale space, molecules are isolated from the outside environment, which allows one to identify and control the source and incidence of chemical reactions. Recent work has demonstrated this new chemistry by using SWCNTs as a nanometer-scale reaction furnace.
As far as test tubes go, it doesn't get any smaller than a single-walled carbon nanotube (SWCNT). Among the wide range of interesting properties exhibited by SWCNTs is their capacity to encapsulate molecules within their quasi one-dimensional cavity. The confinement offered by the nanotube could serve as a nanoscale test tube to constrain a chemical reaction. This was demonstrated in principle back in 1998, when the coalescence of adjacent fullerenes was observed by transmission electron microscopy. In the following years, scientists have extensively experimented with filling nanotubes with other fullerenes, atoms, molecules and, very recently, with organic molecules. Owing to their large variety with diverse chemical properties, the incorporated organic molecules can tune the properties of the SWCNTs. Scientists are intrigued by the possibilities that SWCNTs' use as a reaction tube offers for chemistry at the nanoscale. Nanochemistry - a key to control self-assembly processes prerequisite for nanotechnology - in essence would produce stable chemical reactions inside a confined nanoscale space. Encapsulated inside this nanoscale space, molecules are isolated from the outside environment, which allows one to identify and control the source and incidence of chemical reactions. Recent work has demonstrated this new chemistry by using SWCNTs as a nanometer-scale reaction furnace.
May 9th, 2008
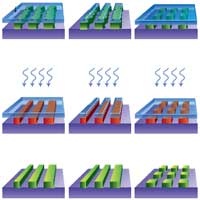 In the past, random defects caused by particle contamination were the dominant reason for yield loss in the semiconductor industry - defects occur in the patterning process (so-called process defects) when contaminants become lodged in or on the wafer surface. Trying to prevent such fabrication defects, chip manufacturers have spent much effort and money to improve the fabrication process, for instance by installing ultra-clean fabrication facilities. With the semiconductor industry's move to advanced nanometer nodes, and feature sizes approaches the limitation of the fabrication method used, particles are no longer the only problem for chip manufacturers. In a nanoscale feature-size fabrication environment, systematic variations, such as metal width and thickness variations or mask misalignment, are also major contributors to yield loss. Rather than perfecting a nanostructure by improving its original fabrication method, researchers at Princeton University have demonstrated a new method, known as self-perfection by liquefaction (SPEL), which removes nanostructure fabrication defects and improves nanostructures after fabrication.
In the past, random defects caused by particle contamination were the dominant reason for yield loss in the semiconductor industry - defects occur in the patterning process (so-called process defects) when contaminants become lodged in or on the wafer surface. Trying to prevent such fabrication defects, chip manufacturers have spent much effort and money to improve the fabrication process, for instance by installing ultra-clean fabrication facilities. With the semiconductor industry's move to advanced nanometer nodes, and feature sizes approaches the limitation of the fabrication method used, particles are no longer the only problem for chip manufacturers. In a nanoscale feature-size fabrication environment, systematic variations, such as metal width and thickness variations or mask misalignment, are also major contributors to yield loss. Rather than perfecting a nanostructure by improving its original fabrication method, researchers at Princeton University have demonstrated a new method, known as self-perfection by liquefaction (SPEL), which removes nanostructure fabrication defects and improves nanostructures after fabrication.
May 4th, 2008
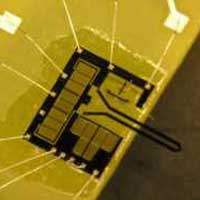 Future nanomanufacturing processes will rely on two basic principles: a combination of chemical synthesis and self-assembly on one hand and robotic nanofabrication on the other. While the former is a controlled 'natural' process relying on chemistry and self-organization principles of nature, the latter will be an industrial process similar in concept to today's automated manufacturing assembly lines. Robotic assembly lines in modern factories have come a long way since the early 20th century when Henry Ford first used an assembly line on an industrial scale for his Model T automobile. Nevertheless, the principle is the same. Rather than having a single craftsman or team of craftsmen create each part of a product individually and assemble them together into a single item, an assembly line is a (often completely automated) manufacturing process in which interchangeable parts are added to a product in a sequential manner to create a finished product. While sporadic automation of certain tasks has already begun (for instance, automated microrobotic injection of foreign materials into biological cells), nanotechnology techniques today are pretty much where the industrial world was before Ford's assembly line - a domain of highly skilled artisans and not of automated mass production. It has long been a dream for nanotechnologists that robots could one day be used in an assembly line type of process to manufacture nanodevices. Researchers are beginning to develop the first rudimentary nanomanipulation devices that could lead to future automated manufacturing systems. Now, a team of scientists in Canada have reported the first demonstration of closed-loop force-controlled grasping at the nanonewton level.
Future nanomanufacturing processes will rely on two basic principles: a combination of chemical synthesis and self-assembly on one hand and robotic nanofabrication on the other. While the former is a controlled 'natural' process relying on chemistry and self-organization principles of nature, the latter will be an industrial process similar in concept to today's automated manufacturing assembly lines. Robotic assembly lines in modern factories have come a long way since the early 20th century when Henry Ford first used an assembly line on an industrial scale for his Model T automobile. Nevertheless, the principle is the same. Rather than having a single craftsman or team of craftsmen create each part of a product individually and assemble them together into a single item, an assembly line is a (often completely automated) manufacturing process in which interchangeable parts are added to a product in a sequential manner to create a finished product. While sporadic automation of certain tasks has already begun (for instance, automated microrobotic injection of foreign materials into biological cells), nanotechnology techniques today are pretty much where the industrial world was before Ford's assembly line - a domain of highly skilled artisans and not of automated mass production. It has long been a dream for nanotechnologists that robots could one day be used in an assembly line type of process to manufacture nanodevices. Researchers are beginning to develop the first rudimentary nanomanipulation devices that could lead to future automated manufacturing systems. Now, a team of scientists in Canada have reported the first demonstration of closed-loop force-controlled grasping at the nanonewton level.
Apr 25th, 2008
 There are several touch sensor technologies available to power touch screens like the ones you can find on your bank ATM, airport check-in kiosk or other self-service terminals. What they all have in common is that they are sensitive to human touch because their screens are coated with a special transparent thin film that act as a sensor. This sensor generally has an electrical current or signal going through it and touching the screen causes a voltage or signal change. Apart from touch screens, transparent conductive thin films are used in numerous products such as flat-panel displays, solar cells or as thermal barriers in energy-saving windows. Future applications will include flexible displays for e-papers, smart cards, 'heads-up' displays integrated into cockpit and car windows, and windows that can be used as a light source at night. All this has driven increased research activity in finding alternative novel transparent electrode materials with good stability, high transparency and excellent conductivity. Graphene is one good candidate and films based on carbon nanotubes have attracted significant attention recently as well. Researchers now have demonstrated the use of metallic nanotubes to make thin films that are semitransparent, highly conductive, flexible and come in a variety of colors.
There are several touch sensor technologies available to power touch screens like the ones you can find on your bank ATM, airport check-in kiosk or other self-service terminals. What they all have in common is that they are sensitive to human touch because their screens are coated with a special transparent thin film that act as a sensor. This sensor generally has an electrical current or signal going through it and touching the screen causes a voltage or signal change. Apart from touch screens, transparent conductive thin films are used in numerous products such as flat-panel displays, solar cells or as thermal barriers in energy-saving windows. Future applications will include flexible displays for e-papers, smart cards, 'heads-up' displays integrated into cockpit and car windows, and windows that can be used as a light source at night. All this has driven increased research activity in finding alternative novel transparent electrode materials with good stability, high transparency and excellent conductivity. Graphene is one good candidate and films based on carbon nanotubes have attracted significant attention recently as well. Researchers now have demonstrated the use of metallic nanotubes to make thin films that are semitransparent, highly conductive, flexible and come in a variety of colors.
Apr 15th, 2008
 Have you ever tried to peel a fresh tomato? Then you probably know that frustrating feeling when you end up with lots of little, mostly triangular pieces of skin. Of course you will also have remembered your grandma's trick to pour hot water over a tomato before skinning it; surprisingly, the skin then comes off easily in just a few large pieces. There are lots of other examples from our daily lives with similarly aggravating experiences: Frustrated by scotch tape that won't peel off the roll in a straight line? Angry at wallpaper that refuses to tear neatly off the wall? Cursing at the price sticker that doesn't come off in one piece? Or you dutifully follow the 'tear along the dotted line' instruction on a re-sealable bag only to be confronted with a tear that is anywhere but on the dotted line. Physicists, mathematicians and materials engineers love these things because it gives them a chance to explain everyday phenomena with impressive looking formulas and diagrams. Wrinkling, folding and crumpling of thin films have been characterized by experiments, theory and numerical simulations. A new study now adds a new element: fracture. The results suggest that the coupling between elasticity, adhesion and fracture, imprinted in a tear shape, can be used to evaluate mechanical properties of thin films and could even be applied at the nanoscale.
Have you ever tried to peel a fresh tomato? Then you probably know that frustrating feeling when you end up with lots of little, mostly triangular pieces of skin. Of course you will also have remembered your grandma's trick to pour hot water over a tomato before skinning it; surprisingly, the skin then comes off easily in just a few large pieces. There are lots of other examples from our daily lives with similarly aggravating experiences: Frustrated by scotch tape that won't peel off the roll in a straight line? Angry at wallpaper that refuses to tear neatly off the wall? Cursing at the price sticker that doesn't come off in one piece? Or you dutifully follow the 'tear along the dotted line' instruction on a re-sealable bag only to be confronted with a tear that is anywhere but on the dotted line. Physicists, mathematicians and materials engineers love these things because it gives them a chance to explain everyday phenomena with impressive looking formulas and diagrams. Wrinkling, folding and crumpling of thin films have been characterized by experiments, theory and numerical simulations. A new study now adds a new element: fracture. The results suggest that the coupling between elasticity, adhesion and fracture, imprinted in a tear shape, can be used to evaluate mechanical properties of thin films and could even be applied at the nanoscale.
Apr 9th, 2008
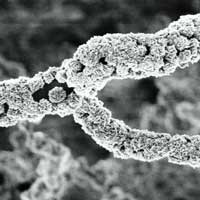 Diamonds have been known in India for at least 3000 years and are thought to have been first recognized and mined there. The most familiar usage of diamonds today is as gemstones in jewelry but, apart from being a girl's best friend, it seems that diamonds, especially nanodiamonds, are quickly becoming a scientist's best friend as well. Diamonds are the hardest natural material - the word diamond comes from the Greek term adamas, which means 'invincible' - has the lowest coefficient of thermal conductivity, is electrically insulating, chemically inert, and optically transparent. In nanoparticulate form, diamonds possess an additional property that makes them so interesting for researchers: since they are carbon-based and non-toxic they are a suitable material for drug delivery, drug diagnostics and medical imaging applications. One of the challenges in fabricating nanodiamond coatings and composite materials is the difficulty of controlling the size, texture, and crystalline quality of the diamond particles. Now, researchers in Portugal have demonstrated for the first time the facile fabrication and the conformal coating of nanocrystalline diamond onto silica nanofibers by a two-step method: synthesis of templates on silicon wafer; and coating of the silica fibers with nanocrystalline diamond.
Diamonds have been known in India for at least 3000 years and are thought to have been first recognized and mined there. The most familiar usage of diamonds today is as gemstones in jewelry but, apart from being a girl's best friend, it seems that diamonds, especially nanodiamonds, are quickly becoming a scientist's best friend as well. Diamonds are the hardest natural material - the word diamond comes from the Greek term adamas, which means 'invincible' - has the lowest coefficient of thermal conductivity, is electrically insulating, chemically inert, and optically transparent. In nanoparticulate form, diamonds possess an additional property that makes them so interesting for researchers: since they are carbon-based and non-toxic they are a suitable material for drug delivery, drug diagnostics and medical imaging applications. One of the challenges in fabricating nanodiamond coatings and composite materials is the difficulty of controlling the size, texture, and crystalline quality of the diamond particles. Now, researchers in Portugal have demonstrated for the first time the facile fabrication and the conformal coating of nanocrystalline diamond onto silica nanofibers by a two-step method: synthesis of templates on silicon wafer; and coating of the silica fibers with nanocrystalline diamond.
Apr 8th, 2008
 Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries.
Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries.
Apr 7th, 2008
 Nanotechnology researchers have appropriated the name of Janus - the Roman god of gates and doorways, usually depicted with two heads looking in opposite directions - to name a class of amphiphilic (i.e. containing both hydrophobic and hydrophilic portions) nanoparticles composed of two fused hemispheres, each made from a different substance. Their particular structure makes Janus particles an intriguing subject for exploring novel anti-cancer therapies where they, for instance, carry two different and complementary medicines. In a novel use of Janus particles, researchers have now isolated a means of using them to make 're-sealable' pores in lipid bilayer membranes. Described in another way, the localization of the nanoparticles in the pore can be thought of as the placement of a zipper, which allows a specific slit to be opened or closed at will.
Nanotechnology researchers have appropriated the name of Janus - the Roman god of gates and doorways, usually depicted with two heads looking in opposite directions - to name a class of amphiphilic (i.e. containing both hydrophobic and hydrophilic portions) nanoparticles composed of two fused hemispheres, each made from a different substance. Their particular structure makes Janus particles an intriguing subject for exploring novel anti-cancer therapies where they, for instance, carry two different and complementary medicines. In a novel use of Janus particles, researchers have now isolated a means of using them to make 're-sealable' pores in lipid bilayer membranes. Described in another way, the localization of the nanoparticles in the pore can be thought of as the placement of a zipper, which allows a specific slit to be opened or closed at will.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





