
Latest Headlines
![]()
Scientists grow human mini-lungs as animal alternative for nanomaterial safety testing

Human mini-lungs grown by scientists can mimic the response of animals when exposed to certain nanomaterials. Though not expected to replace animal models completely, human organoids could soon lead to significant reductions in research animal numbers.
April 18, 2024
![]()
An ink for 3D-printing flexible devices without mechanical joints

Researchers are targeting the next generation of soft actuators and robots with an elastomer-based ink for 3D printing objects with locally changing mechanical properties, eliminating the need for cumbersome mechanical joints.
April 18, 2024
![]()
Researchers can now measure important thermal properties of ultrathin silicon membranes
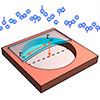
Measuring thermal properties of ultrathin silicon membranes without physical contact enables pristine characterization for advanced chip manufacturing.
April 18, 2024
![]()
MXene nanogenerator device harnesses sweat power for fitness trackers

A small amount of sweat could be all that's needed to power fitness trackers of the future.
April 18, 2024
![]()
Enhancing chemical production with enzyme-packed flow-through reactors using tailor-made nanomembranes

Enzyme-packed flow-through reactors with custom nanomembranes significantly enhance chemical production yields and reaction rates for sustainable applications.
April 18, 2024
![]()
New understanding of energy losses in emerging light source

Researchers achieved a breakthrough in the development of next-generation light sources with a new method for understanding and measuring efficiency losses in light-emitting electrochemical cells.
April 18, 2024
![]()
![]()
Metal-organic framework coatings enable simple, reusable water contaminant testing

Coating glass vials with metal-organic frameworks enables simple, rapid, reusable and highly sensitive detection of water contaminants, outperforming current methods.
April 18, 2024
![]()
![]()
AI tool predicts responses to cancer therapy using information from each cell of the tumor

Study showcases the power of machine learning to predict therapy outcomes using rich information from single-cell omics .
April 18, 2024
![]()
Atom-by-atom: Imaging structural transformations in 2D materials

In an effort to understand how and why 2D interfaces take on the structures they do, researchers have developed a method to visualize the thermally-induced rearrangement of 2D materials, atom-by-atom, from twisted to aligned structures using transmission electron microscopy (TEM).
April 18, 2024
![]()
Two-dimensional nanomaterial sets record for expert-defying, counter-intuitive expansion

When stretched in one direction, nanomaterial expands perpendicular to applied force.
April 18, 2024
![]()
![]()
A simpler way to inorganic perovskite solar cells

New research reveals that air annealing enhances the optoelectronic properties of CsPbI3 perovskite solar cells, reducing defects and potentially simplifying mass production.
April 17, 2024
![]()
![]()
Broadband gold nanogap sensor revolutionizes material testing and virus detection

Broadband gold nanogap sensor enables rapid, precise detection of materials and viruses, enhancing pandemic preparedness with real-time, high-sensitivity results.
April 17, 2024
![]()
A new spin on materials analysis

Electron spin states can now be probed at much higher resolution and more efficiently, opening new opportunities in materials analysis and data processing technologies.
April 17, 2024
![]()
Tracking a protein's fleeting shape changes
Researchers have developed a powerful, new technique to generate 'movies' of changing protein structures and speeds of up to 50 frames per second.
April 17, 2024
![]()
![]()
Paintable 'second skin' gel for wearable bioelectronic sensors
Researchers have developed an on-skin paintable waterproof biohydrogel for wearable bioelectronics, enabling high-fidelity monitoring of bioelectrical signals like ECG.
April 17, 2024
![]()
Unlocking multi-color ultra-long phosphorescence with carbonized polymer nanodots

Overview of a synthesis method for carbonized polymer nanodots that produce multi-color, ultra-long room-temperature phosphorescence.
April 17, 2024
![]()
From defects to order: Spontaneously emerging crystal arrangements in perovskite halides

Scientists discovered a new defect-ordered layered halide perovskite, shedding light on how order can emerge through defects in these compounds.
April 17, 2024
![]()
