| Posted: Sep 29, 2017 | |
Monitoring oxygen reaction dynamics with an individual nanosheet-based electronic circuit |
|
| (Nanowerk Spotlight) Water electrolysis has been regarded as an environmentally friendly route to hydrogen gas production. However, efficiency of water catalysis is severely limited by poor kinetics of the oxygen evolution reaction (OER). In-depth understanding towards this reaction is expected to be a fundamental guideline for achieving a high-efficiency catalysis system design. | |
| It has been challenging to make a real-time monitor of this interfacial reaction process. Difficulties arise from traditional measurement limitations, for example, the active species are generally mixed with binders or conductive additives, and interfaces for catalytic reaction are buried between solid and liquid phases, which are difficult to access and detect by conventional techniques. | |
| "Previously, most of fundamental OER studies focused on the relationships between materials’ catalytic activities and their electronic structure, aided by a wealth of spectroscopic techniques and first-principles computations," Liqiang Mai, a professor at the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology (WUT), tells Nanowerk. "However, there are much fewer studies on OER kinetic processes, particularly those processes taking place in an electrode/electrolyte interface region. The distribution of ions and water at the interface determine the kinetics of mass and electron transfer process. Our understanding is still quite limited." | |
| In the new work published in Nature Communications ("Oxygen evolution reaction dynamics monitored by an individual nanosheet-based electronic circuit"), Mai and his team have designed a single electrochemical nanodevice, which consist of nickel nanoparticle catalysts anchored on a graphene nanosheet electrode (Ni-graphene). | |
| They proposed and used an in situ measurement of electrode conductivity to probe the effect of O2 on the OER kinetic process for the first time. A significant decrease (over 20%) in Tafel slope and an early onset potential of 1.344 V (with reference to RHE) are observed by removing O2 in the electrolyte. | |
 |
|
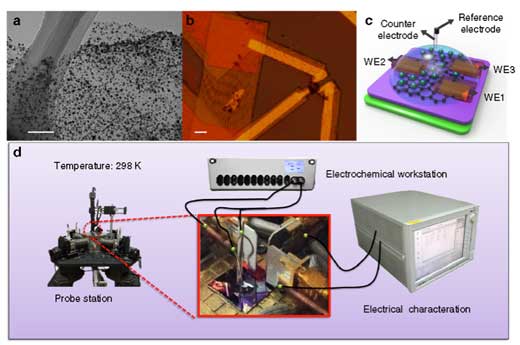
| Working principle of temporal electrical transport measurement. (a) TEM image of as-prepared Ni-graphene nanosheets with a scale bar at 200 nm. (b) Optical microscope image of nanosheets contacted with three metallic electrodes with a scale bar at 10 µm. (c) Schematic illustration of the Ni-graphene nanosheet-based device with a microscopic electrochemical cell on it. (d) Illustration of the device (inset) and corresponding measurement equipment layout with the three-dimensional view. (Reprinted with permission by Nature Publishing Group) (click on image to enlarge) | |
| Mai notes that "several unique advantages enable such a nanodevice to be a great platform to directly probe the OER processes for reliable information. First, a single nanowire/nanosheet can be used as the working electrode, avoiding influences of the binders and conductive carbon additives." | |
| "Second, structure and composition of the nanodevice can be precisely designed and controlled, which is essential to eliminate experimental uncertainties and allows quantitatively or semi-quantitatively fundamental studies." | |
| "Third, it is much easier to monitor the change of physical properties of individual nanowire and nanosheet (such as conductivity) in a nanodevice. This advantage is largely unexplored up to date. It could allow us to probe interface properties during the OER process directly." | |
| "Oxygen is a product of OER. In terms of reaction equilibrium (i.e., Le Chatelier’s principle), it is known that O2 concentration increase in electrolyte (with the ongoing of OER) hinders the catalytic reaction. But there is no clear answer whether oxygen molecules would affect OER kinetics," Peiyao Wang, a Ph.D. candidate who worked on this project, points out. "It is interesting and reasonable to expect that oxygen molecules could adsorb at the reaction interfaces in consideration of some intermolecular forces, hindering the formation of hydroxide and hence the catalytic kinetic performance." It is the hypothesis of this project. | |
| The mechanism of the O2 inhibition effect has been investigated further by cooperating with Zhe Liu’s group, at the University of Melbourne. Through molecular dynamics (MD) simulations, they found that the detrimental effect of oxygen should stem from the strong van der Waals (vdW) force between O(O2) and Ni/NiO, which is nearly the same to the interaction between O(OH-) and Ni/NiO. The similar interaction force drives the O2 and OH- occupy similar positions next to the Ni/NiO surface. This reduces the amount of OH- in the double layer and thus resulting in a poor kinetics and negative catalytic performance. | |
| Different from the Le Chatelier's principle, which provides a clue to understand reaction direction from thermodynamic perspective, the research reported in this paper provides insights on how surface adsorbed O2 could influence the reaction kinetics. | |
| "We expect the results from this work to be valuable pointers towards the design of high-performance catalyst systems. This work also presents a powerful nano-electrochemical device platform and a temporal I-V measurement method to investigate the kinetic interface properties," concludes Mai. | |
| In the future, the researchers hope to optimize the catalyst system structure, designing a continuous and effective oxygen elimination catalyst system, and achieving a high efficiency of OER process for practical application. | |
|
Provided by State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, as a Nanowerk exclusive.
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
