Nanoparticles, free radicals and oxidative stress
Free radicals are unstable atoms or molecules with free outer electrons. This makes them highly reactive because free electrons always strive to form a stable bond. This stabilization involves gaining an electron from another molecule, triggering a chain reaction. Such reactions are omnipresent in the human body, but under certain circumstances can damage biomolecules. Whether nanoparticles are intracellular taken up leading to the activation of free radical production is currently being discussed.
Ongoing studies are investigating whether the amount of free radicals formed on the surface of nanoparticles is sufficient to induce cellular effects. This dossier provides an overview about what free radicals are, how they originate, why organisms need them, how they are neutralized, and what we know about the connection between nanoparticles and free radical production.
Introduction
There is consensus that the surface structure of nanoparticles plays a crucial role in the interaction with cells and should therefore be considered when evaluating their health effects. The discussion often centers around mechanisms of action involving free radical development after nanoparticle uptake into the cell.
Free radicals play an important role in oxygen-dependent (aerobic) living systems. They are an important component in cell respiration and other vital cellular processes, but are also involved in aging and in disease development.
Free radicals are unstable molecules with free outer electrons (unpaired electrons). They are highly reactive because the free electrons always strive to bond with other electrons and form covalent pairs. In this process the free radicals strip the electrons from other molecules. This chain reaction is omnipresent in every cell of the human body. Beyond affecting cellular regulation, it can also damage molecules such as carbohydrates, fats, proteins and nucleic acids.
What are free radicals?
Free radicals are atoms or molecules that contain one or more unpaired electrons and are, in this sense, free. This makes many free radicals highly reactive, i.e. they have a strong tendency to form pairs to counteract the labile unpaired condition.
To this end, the free radicals gain electrons from any available donor or donate an electron to a suitable acceptor, which in turn become modified into a secondary free radical. This chain reaction can cause biological damage (Fig. 1).
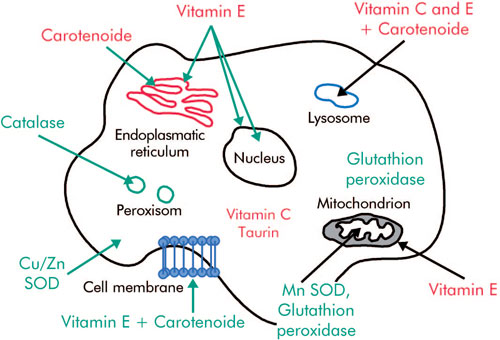
There are many different sources of free radicals within cells and the environment. In aerobic organisms, free radicals are produced during and through normal metabolic processes.
Key sources include electron transfer in the plasma membrane and cell respiration in the mitochondrial membrane. The production can proceed enzymatically (with catalysts) or non-enzymatically.
One research team claims3 that the mitochondria are the main source of the oxidative damage because free radicals such as superoxide can escape from the electron transport chain.
About 3-10 % of the oxygen turned over there is not fully processed, i.e. reduced. They can enter the cytosol of the cell, where free radicals can react with other substances and thereby form new radicals by removing electrons from these substances. This triggers a chain reaction in which electrons change their owners and that ends in cellular changes such as DNA modification or enzyme disruption.
Antioxidants – oxidative stress
In order to counteract intracellular damage by free radicals, cells have developed a so-called intracellular antioxidant system. This process transforms free electrons into a nonreactive form by proteins (enzymes).
Antioxidants regulate oxidative reactions by inhibiting, delaying or hampering the oxidation of the substances4. The intracellular enzymes function as antioxidants are the backbone of this cellular defense system5,6.
The key antioxidant enzymes possess certain elements that shield and protect proteins7,8.
Nonenzymatic antioxidants can also neutralize radicals4 (e.g. water-soluble substances such as vitamin C, glutathione or fat-soluble substances such as vitamin E or vitamin A/βcarotene).
For example, the enzyme SOD transforms superoxide radicals into hydrogen peroxide, which is then broken down by catalysis into water and oxygen.
Free radicals are not exclusively damaging metabolic products, but also have a series of important functions. For example they serve in immune defense because leucocytes and macrophages utilize their bactericidal effects: they produce free radicals and thus destroy bacteria and other foreign substances.
Moreover, free radicals probably play a role in the bodys tumor suppression by mediating programmed cell death (apoptosis).
Immune-relevant cells also use the reactive potential of ROS as a cellular defense mechanism against entering pathogens to kill bacteria, viruses and degenerated cells.
Radicals also fulfill important physiological functions such as regulating the vascular tone and those cell functions controlled by oxygen concentration. They also influence signal transmission mechanisms and trigger oxidative stress responses as well as apoptosis11.
The capacity of cellular defense mechanisms is limited. Oxidative stress can therefore lead to malfunction and even to cell death. Oxidative stress is the result of an imbalance between the intracellular production of free radicals and the cellular defense mechanisms.
The balance between oxidants and antioxidants can be disrupted by an increase in free radicals or a reduction of anti-oxidative substances. Oxidative stress can trigger a number of potentially damaging biochemical reactions9.
Studies show that the production of radicals is directly involved in the oxidative destruction of macromolecules such as lipids, proteins and nucleic acids. Under certain conditions, phagosomes (vesicles in which bacteria, for example, are taken up)10 of macrophages can degenerate and release their contents into other cell compartments, damaging DNA through oxidative reactions.
Chronic infections can therefore trigger chronic inflammatory reactions by promoting permanent phagocytic activity of macrophages.
Further on, chronic inflammation is known to be a risk for cancer induction.
Moreover, free radicals play a role in many degenerative diseases and cell aging processes3.
The oxidation and reduction balance in cells (redox homeostasis)
Free radicals and their derivates, along with reactive non-radicals that can be attributed to radicals, are always present in living systems in relatively low and balanced amounts.
The concentration of free radicals depends on their production and their clearance. Clearance is controlled by various enzymes and non-enzymatic antioxidants (for example vitamins E, A, C and glutathione).
Cells are in a stable state when the rate of ROS production and the anti-oxidation capacity are in balance. This is referred to as a balanced redox capacity. This balance can be disrupted either by increased ROS production or by the reduced capacity of the antioxidants.
As free radicals can donate an electron to a suitable receptor (reduction reaction) or can bond their unpaired electron with a suitable donor (oxidation reaction), free radicals play an important role in maintaining the redox balance in cells.
Depending on the duration and strength of the imbalance (which can be temporally limited), the redox regulation of the cell fulfills a compensatory function. This physiological mechanism is termed redox homeostasis.
When, however, a constant production of free radicals is triggered, for example by oxidative stress, then the redox homeostasis becomes unbalanced because the cellular mechanisms are no longer capable of establishing the normal levels. This can persistently change signal transmission, but also lead to changes on gene and protein levels of the cells and thus promote so-called oxidative conditions or processes.
This includes virtually all complex molecules that can gain a single electron (DNA, proteins, lipids and carbohydrates) and thus be damaged by highly reactive radicals. When ROS is consistently elevated over a longer time period (chronic conditions) free radicals can cause damage and lead to pathological conditions.
Nanoparticles and free radicals
Various in vitro and in vivo studies show that free radical formation can be triggered by nanoparticles (fullerenes, carbon nanotubes, quantum dots, emission particles)12,13. Nanoparticles can be taken up actively (phagocytosis) by certain cells (macrophages) and initiate ROS formation14, 15. Passive cellular uptake of particles has also been documented.
The decisive question, however, is whether more ROS is formed per cell when more particles are taken up. Nanoparticles tend to form aggregations/agglomerations. It is unclear whether they can produce elevated ROS levels in this configuration.
ROS can also develop directly on the surface of the particles, although this depends on particle structure (metallic particles, for example, function as catalysts). Due to the larger surface area to mass ratio of smaller particles, more ROS could be formed than in larger particles16,17. It remains unknown whether aggregation influences the amount of ROS formed.
The overproduction or chronic production of reactive oxygen species can cause inflammatory reactions, tissue changes and DNA, protein and lipid damage. Nanoparticles also cause mechanical damage within the cells and thus trigger oxidative stress.
To a certain degree, antioxidants can neutralize the free radicals through homeostatic activity of the cells. If more ROS is generated than neutralized, then this system can shift and certain biomolecules become oxidized and/or altered.
In a pilot study18 a special mouse model was used to show that long (ca. 20 µm), needle-shaped nanotubes administered into the abdominal cavity (intraperitoneally) caused chronic inflammations, whereas short and/or curved as well as long, curved nanotubes induced no such effects.
As these tubes closely resemble asbestos fibers in their structure (form, length and solubility), a comparable mechanism of action is being discussed. Asbestos exposure can lead to so-called mesotheliomas (connective tissue tumors) in the pleura area.
Such tumors are not necessarily malignant. They can develop when macrophages attempt to ingest the uptaken needle-shaped fibers. These cells are not succeeding since the fibers are too long.
The presence of free radicals is therefore accompanied by the formation of so-called giant cells, because several cells fuse with each other in order to successfully ingest the fibers. The chronic activation of these cells leads to the development of nodular new tissue formation, so-called granulomas. Over time, these can develop into mesotheliomas.
The above-mentioned study specifically used nanotubes that resembled asbestos fibers in form and length. Other fibers, however, were also tested in order to compare their effects. As expected, the results showed that only the long, needle-shaped nanotubes, not the short and/or curved ones, triggered chronic inflammation (granulomas).
An important consideration in evaluating this study is the relatively high concentration of nanotubes (50 µg) per mouse and the very specific animal model used. The study itself also emphasizes the differences in the nanotubes with regard to the pre-treatments.
Furthermore to assess the significance of this study the experimental conditions and the limited number of the used animals has to be considered. While the information from this study must be taken seriously, it needs to be verified and reproduced.
Conclusions
Nanoparticles that are taken up intracellular can induce cellular effects whose biological relevance remains to be clarified.
Experimental studies show that nanoparticles can trigger the production of free radicals. The chronic release of such reactive molecules can lead to tissue degeneration.
Most studies have tested very high concentrations of nanoparticles over relatively short exposure times. Such data hamper a definitive health risk assessment. The homeostatic activity of cells and organisms counteracts exposure to nanoparticles.
It remains unclear at which point the system becomes unbalanced, leading to biological and health-related impacts. This calls for conducting targeted, standardized and dose-dependent long-term studies on the underlying mechanisms.
Notes and References
3 Shigenaga, M. K., Hagen, T. M. and Ames, B. N., 1994, Oxidative damage and mitochondrial decay in aging, PNAS 91(23), 10771-8.
4 Sies, H., 1997, Oxidative stress: oxidants and antioxidants, Exp Physiol 82(2), 291-5.
5 Dreher, D., Jornot, L. and Junod, A. F., 1995, Effects of hypoxanthine-xanthine oxidase on Ca2+ stores and protein synthesis in human endothelial cells, Circ Res 76(3), 388-95.
6 Dreher, D. and Junod, A. F., 1995, Differential effects of superoxide, hydrogen peroxide, and hydroxyl radical on intracellular calcium in human endothelial cells, J Cell Physiol 162(1), 147-53.
7 Harris, E. D., 1992, Regulation of antioxidant enzymes, Faseb J 6(9), 2675-83.
8 Harris, E. D., 1992, Copper as a cofactor and regulator of copper, zinc superoxide dismutase, J Nutr 122(3 Suppl), 636-40.
9 Droge, W., 2002, Free radicals in the physiological control of cell function, Physiol Rev 82(1), 47-95.
10 Simkó, M., Fiedeler, U., Gazsó, A. and Nentwich, M., 2008, Einfluss von Nanopartikeln auf zelluläre Funktionen. NanoTrust-Dossiers Nr. 007en, hrsg. v. Institut für Technikfolgen-Abschätzung, Wien.
11 Simkó, M., 2007, Cell type specific redox status is responsible for diverse electromagnetic field effects, Curr Med Chem 14(10), 1141-52.
12 Raab, C., Simkó, M., Gazsó, A., Fiedeler, U. and Nentwich, M., 2008, Was sind synthetische Nanopartikel?, NanoTrust-Dossiers Nr. 002, hrsg. v. Institut für Technikfolgen-Abschätzung, Wien.
13 Oberdorster, G., Oberdorster, E. and Oberdorster, J., 2005, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles, Environ Health Perspect 113(7), 823-39.
14 Brown, D. M., Donaldson, K., Borm, P. J., Schins, R. P., Dehnhardt, M., Gilmour, P., Jimenez, L. A. and Stone, V., 2004, Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles, Am J Physiol Lung Cell Mol Physiol 286(2), L344-53.
15 Risom, L., Lundby, C., Thomsen, J. J., Mikkelsen, L., Loft, S., Friis, G. and Moller, P., 2007, Acute hypoxia and reoxygenation-induced DNA oxidation in human mononuclear blood cells, Mutat Res 625(1-2), 125-33.
16 Sioutas, C., Delfino, R. J. and Singh, M., 2005, Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research, Environ Health Perspect 113(8), 947-55.
17 Stone, V., Tuinman, M., Vamvakopoulos, J. E., Shaw, J., Brown, D., Petterson, S., Faux, S. P., Borm, P., MacNee, W., Michaelangeli, F. and Donaldson, K., 2000, Increased calcium influx in a monocytic cell line on exposure to ultrafine carbon black, Eur Respir J 15(2), 297-303.
18 Poland, C. A., Duffin, R., Kinloch, I., Maynard, A.,Wallace, W. A., Seaton, A., Stone, V., Brown, S., Macnee, W. and Donaldson, K., 2008, Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study, Nat Nanotechnol 3(7), 423-8.
Source: NanoTrust, Austrian Academy of Sciences. NanoTrust Dossiers are published irregularly and contain the research results of the Institute of Technology Assessment in the framework of its research project NanoTrust.
