ALD (Atomic Layer Deposition)
Definition: Atomic Layer Deposition (ALD) is a thin film deposition technique that allows for the precise control of the film thickness at the atomic level, layer by layer.
Principle of Operation
ALD is based on the sequential use of a gas phase chemical process. The ALD cycle consists of four main steps: (1) pulsing of a precursor A, (2) purging of the reactor to remove excess precursor and reaction byproducts, (3) pulsing of a second precursor B, and (4) another purging step. These steps are repeated until the desired film thickness is achieved. The unique aspect of ALD is the self-limiting nature of the chemical reactions, ensuring that only a single atomic layer is deposited during each cycle. This leads to exceptional uniformity and conformality, even on high-aspect-ratio structures.
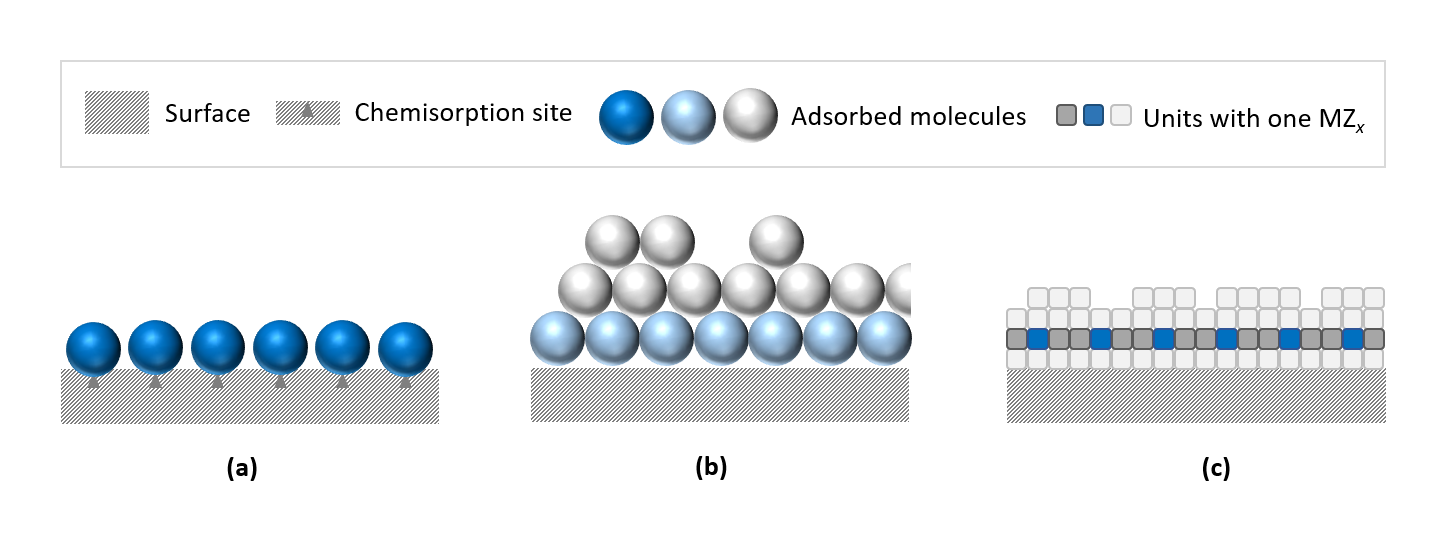
Key Features of the ALD Process
- Atomic Scale Control: ALD allows for precise control over film thickness and composition at the atomic scale.
- Uniformity and Conformality: Films deposited by ALD are extremely uniform and conformal, making it ideal for coating complex 3D structures.
- Material Variety: ALD can deposit a wide range of materials, including oxides, sulfides, nitrides, and metals.
- Low Temperature Processing: Many ALD reactions can occur at relatively low temperatures, making it suitable for temperature-sensitive substrates.
Limitations
Despite its advantages, ALD has some limitations:
- Slow Deposition Rates: The layer-by-layer growth mechanism makes the deposition process slower than other thin film deposition techniques.
- Dependency on Precursor Chemistry: The success of an ALD process highly depends on the availability and reactivity of the precursor chemicals. Finding precursors that are both sufficiently volatile for easy delivery into the reaction chamber and reactive at the desired temperatures, without causing unwanted side reactions, can be challenging. This complexity often requires extensive research and development to identify effective precursor combinations that meet specific material quality and deposition condition requirements.
- Equipment Cost: The specialized equipment required for ALD can be expensive, limiting its accessibility for some applications.
Applications
ALD is utilized in a variety of fields due to its unique advantages:
- Electronics and Semiconductors: For the fabrication of gate oxides, dielectrics, and other critical components in microelectronics.
- Photovoltaics: In the deposition of thin films for solar cells, improving their efficiency and durability.
- Medical Devices: To create biocompatible coatings that can improve the performance and longevity of implants.
- Catalysis: For the preparation of catalysts with high surface areas and controlled compositions.
Further Reading
Biosensors and Bioelectronics, Atomic layer deposition for biosensing applications
Journal of Applied Physics, Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends
Applied Physics Review, Conformality in atomic layer deposition: Current status overview of analysis and modelling
