Cryo-Electron Microscopy: Unveiling the Structure of Biomolecules at Atomic Resolution
What is Cryo-Electron Microscopy?
Cryo-electron microscopy (cryo-EM) is a powerful technique used to determine the three-dimensional structure of biological macromolecules, such as proteins and viruses, at near-atomic resolution. Unlike traditional X-ray crystallography, cryo-EM does not require the formation of crystals, making it suitable for studying large, flexible, and heterogeneous biomolecules. This technique has revolutionized structural biology, enabling researchers to visualize the intricate details of previously challenging targets.
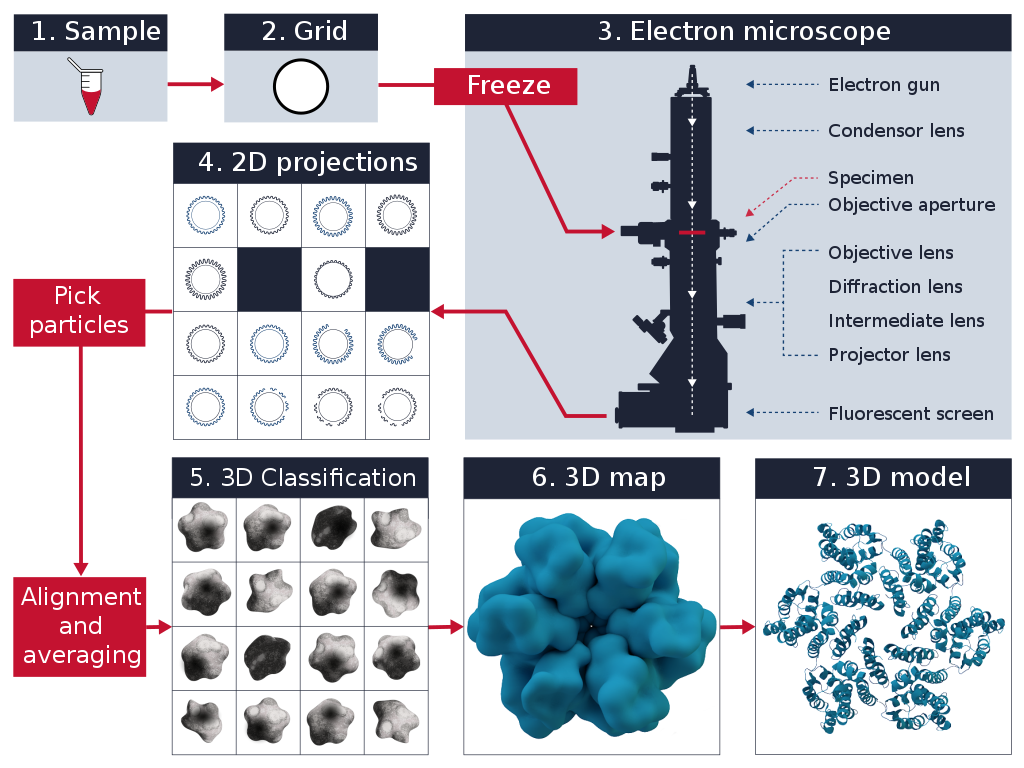
Cryo-EM Workflow
The cryo-EM workflow involves several key steps:
- Sample Preparation: The biological sample is purified and applied to a specialized grid coated with a holey carbon film. The grid is then rapidly plunged into liquid ethane, which is cooled by liquid nitrogen. This rapid freezing process, known as vitrification, preserves the sample in a thin layer of amorphous ice, capturing its native structure.
- Data Collection: The vitrified sample is loaded into a transmission electron microscope (TEM) equipped with a cryo-holder. The microscope operates at cryogenic temperatures to maintain the sample's vitreous state. Electrons are accelerated and focused onto the sample, generating 2D projection images from different orientations. These images are recorded using a direct electron detector.
- Image Processing: The collected 2D projection images undergo extensive computational processing. This includes motion correction to account for sample movement during imaging, contrast transfer function (CTF) estimation and correction, and particle picking to identify individual particles within the images. The selected particles are then aligned and classified based on their orientations and conformations.
- 3D Reconstruction: The aligned and classified particles are used to reconstruct a 3D model of the biomolecule. This is achieved through iterative refinement processes, such as single particle analysis or tomography. The resulting 3D map represents the electron density distribution of the biomolecule, revealing its detailed structure at near-atomic resolution.
- Model Building and Interpretation: The obtained 3D map is used to build an atomic model of the biomolecule. This involves fitting the amino acid sequence into the electron density map and refining the model using computational tools. The final model provides valuable insights into the biomolecule's structure, function, and interactions.
Advantages of Cryo-EM
Cryo-EM offers several advantages over traditional structural biology techniques:
- No Crystallization Required: Cryo-EM does not require the formation of well-ordered crystals, which is a major bottleneck in X-ray crystallography. This allows for the study of large, flexible, and heterogeneous biomolecules that are difficult or impossible to crystallize.
- Near-Native State: The rapid vitrification process in cryo-EM preserves the sample in a near-native state, capturing its physiological conformation. This is particularly important for understanding the function and dynamics of biomolecules in their natural environment.
- High Resolution: Advances in detector technology and image processing algorithms have enabled cryo-EM to achieve near-atomic resolution, rivaling the quality of X-ray crystallography. This high resolution allows for the visualization of individual amino acid side chains and the identification of ligand binding sites.
- Conformational Heterogeneity: Cryo-EM can capture multiple conformational states of a biomolecule within a single sample. By classifying and reconstructing these different conformations, researchers can gain insights into the dynamic nature of biomolecules and their functional mechanisms.
Applications of Cryo-EM
Cryo-EM has revolutionized structural biology and has been applied to a wide range of biological systems:
Protein Structure Determination
Cryo-EM has been extensively used to determine the structure of proteins, including membrane proteins, enzymes, and macromolecular complexes. By elucidating the atomic details of these proteins, researchers can gain insights into their function, regulation, and potential drug targets.
Virus Structure and Vaccine Development
Cryo-EM has been instrumental in studying the structure of viruses, including HIV, influenza, and SARS-CoV-2. Understanding the molecular architecture of viruses aids in the development of effective vaccines and antiviral therapies.
Drug Discovery and Design
The high-resolution structures obtained through cryo-EM provide valuable information for drug discovery and design. By visualizing the binding sites of potential drug targets, researchers can rationally design small molecules that specifically interact with these targets, leading to the development of more effective and selective therapeutics.
Future Perspectives
Cryo-EM continues to evolve and improve, with advancements in sample preparation, data collection, and image processing techniques. The development of next-generation electron detectors and the integration of machine learning algorithms are expected to further enhance the resolution and throughput of cryo-EM. Additionally, the combination of cryo-EM with other techniques, such as mass spectrometry and computational modeling, will provide a more comprehensive understanding of biomolecular structure and function.
As cryo-EM becomes more accessible and automated, it is anticipated to become a routine tool in structural biology laboratories worldwide. The ability to study previously intractable targets and capture dynamic processes will open up new avenues for basic research and drug discovery, ultimately advancing our understanding of biological systems at the molecular level.
Further Reading
Nature Reviews Drug Discovery, Cryo-EM in drug discovery: achievements, limitations and prospects
Molecular Cell, Cryo-EM in molecular and cellular biology
Annual review of Biochemistry, Better, Faster, Cheaper: Recent Advances in Cryo???Electron Microscopy
