Mass Spectrometry: Unveiling the Secrets of Molecules
What is Mass Spectrometry?
Mass spectrometry (MS) is an analytical technique used to identify and quantify molecules based on their mass-to-charge ratio (m/z). It provides valuable information about the chemical composition, structure, and purity of a wide range of substances, from small molecules to large biomolecules like proteins. MS has become an indispensable tool in various fields, including chemistry, biology, environmental science, and materials science.
Principles of Mass Spectrometry
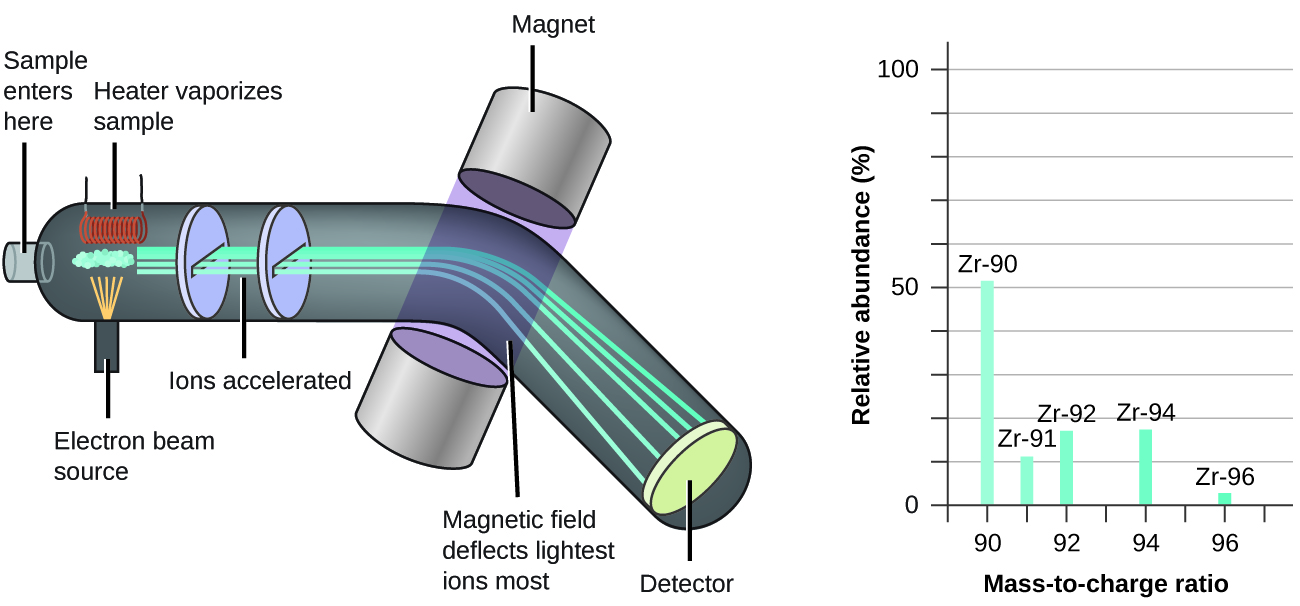
The basic principle of mass spectrometry involves three main steps:
- Ionization: The sample molecules are converted into gas-phase ions, typically by removing or adding electrons or protons. Various ionization techniques are used depending on the nature of the sample, such as electron ionization (EI), electrospray ionization (ESI), and matrix-assisted laser desorption/ionization (MALDI).
- Ion Separation: The generated ions are separated based on their mass-to-charge ratio (m/z) using a mass analyzer. Different types of mass analyzers are employed, including quadrupole, time-of-flight (TOF), ion trap, and Fourier transform ion cyclotron resonance (FT-ICR). Each analyzer has its own strengths and limitations in terms of mass range, resolution, and sensitivity.
- Detection: The separated ions are detected and their abundance is measured by a detector, such as an electron multiplier or a Faraday cup. The detector converts the ion current into an electrical signal, which is then processed to generate a mass spectrum. The mass spectrum plots the relative abundance of ions against their m/z values.
Applications of Mass Spectrometry
Mass spectrometry finds applications in a wide range of fields, including:
Proteomics
MS is a key tool in proteomics, the large-scale study of proteins. It enables the identification and quantification of proteins in complex biological samples, such as cell lysates or tissue extracts. MS-based proteomics techniques, like peptide mass fingerprinting and tandem mass spectrometry (MS/MS), allow researchers to characterize protein sequences, post-translational modifications, and protein-protein interactions.
Metabolomics
Metabolomics focuses on the comprehensive analysis of small molecules (metabolites) in biological systems. MS is widely used to profile metabolites in various samples, such as blood, urine, and cell extracts. By comparing metabolite profiles between different conditions (e.g., healthy vs. diseased), researchers can identify potential biomarkers and gain insights into metabolic pathways and disease mechanisms.
Drug Discovery and Development
MS plays a crucial role in drug discovery and development by providing information about the structure, purity, and pharmacokinetics of drug candidates. It is used to screen compound libraries, identify lead compounds, and assess drug metabolism and stability. MS is also employed in the quality control of pharmaceutical products to ensure their purity and consistency.
Environmental Analysis
MS is a powerful tool for environmental monitoring and analysis. It can detect and quantify a wide range of pollutants, such as pesticides, herbicides, and industrial chemicals, in various environmental matrices (e.g., water, soil, and air). MS-based techniques, like gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS), enable the identification of trace-level contaminants and the assessment of their environmental impact.
Advances in Mass Spectrometry
Mass spectrometry has undergone significant advancements in recent years, leading to improved sensitivity, resolution, and throughput. Some notable developments include:
- High-Resolution Mass Spectrometry: Techniques like FT-ICR and Orbitrap MS offer ultra-high resolution and mass accuracy, enabling the precise determination of elemental compositions and the differentiation of closely related compounds.
- Imaging Mass Spectrometry: Imaging MS techniques, such as MALDI imaging and secondary ion mass spectrometry (SIMS), allow the spatial mapping of molecules directly on tissue sections or surfaces. This provides valuable information about the distribution and localization of compounds in biological and material samples.
- Ion Mobility-Mass Spectrometry: The combination of ion mobility spectrometry with mass spectrometry adds an additional dimension of separation based on the shape and size of ions. This enhances the resolution and enables the differentiation of isomeric compounds.
Challenges and Future Perspectives
Despite the remarkable capabilities of mass spectrometry, several challenges remain. One major challenge is the complexity of data analysis, especially in untargeted analyses like metabolomics and proteomics. The vast amount of data generated requires advanced computational tools and workflows for efficient data processing, integration, and interpretation.
Another challenge is the need for improved sample preparation and ionization techniques to expand the range of analyzable compounds and enhance sensitivity. The development of novel ionization methods and sample introduction systems will be crucial for tackling challenging samples and detecting low-abundance molecules.
Future advancements in mass spectrometry are expected to focus on increasing the speed, sensitivity, and resolution of analyses. The integration of MS with other analytical techniques, such as chromatography and spectroscopy, will provide complementary information and enable more comprehensive characterization of complex samples. Additionally, the application of artificial intelligence and machine learning algorithms will facilitate data analysis and interpretation, opening up new avenues for biomarker discovery and precision medicine.
Further Reading
Drug Discovery Today: Technologies, Recent advances in mass-spectrometry based proteomics software, tools and databases
Analytical and Bioanalytical Chemistry, Recent advances in mass spectrometry imaging of single cells
Frontiers in Nutrition, A review on recent advances in mass spectrometry analysis of harmful contaminants in food
