| Posted: Aug 14, 2014 |
Novel chip-based platform could simplify measurements of single molecules
|
|
(Nanowerk News) Researchers at UC Santa Cruz have developed a new approach for studying single molecules and nanoparticles by combining electrical and optical measurements on an integrated chip-based platform. In a paper published July 9 in Nano Letters ("Correlated Electrical and Optical Analysis of Single Nanoparticles and Biomolecules on a Nanopore-Gated Optofluidic Chip"), the researchers reported using the device to distinguish viruses from similarly-sized nanoparticles with 100 percent fidelity.
|
|
Combining electrical and optical measurements on a single chip provides more information than either technique alone, said corresponding author Holger Schmidt, the Kapany Professor of Optoelectronics in the Baskin School of Engineering and director of the W. M. Keck Center for Nanoscale Optofluidics at UC Santa Cruz. Graduate student Shuo Liu is first author of the paper.
|
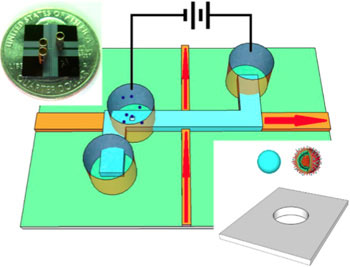 |
| The nanopore-gated optofluidic chip is able to distinguish influenza viruses from nanobeads.
|
|
The new chip builds on previous work by Schmidt's lab and his collaborators at Brigham Young University to develop optofluidic chip technology for optical analysis of single molecules as they pass through a tiny fluid-filled channel on the chip. The new device incorporates a nanopore that serves two functions: it acts as a "smart gate" to control the delivery of individual molecules or nanoparticles into the channel for optical analysis; and it allows electrical measurements as a particle passes through the nanopore.
|
|
"The nanopore delivers a single molecule into the fluidic channel, where it is then available for optical measurements. This is a useful research tool for doing single-molecule studies," Schmidt said.
|
|
Biological nanopores, a technology developed by coauthor David Deamer and others at UC Santa Cruz, can be used to analyze a DNA strand as it passes through a tiny pore embedded in a membrane. Researchers apply voltage across the membrane, which pulls the negatively charged DNA through the pore. Current fluctuations as the DNA moves through the pore provide electrical signals that can be decoded to determine the genetic sequence of the strand.
|
|
With the new device, researchers are able to gather electrical measurements on a nanoparticle as it moves through a pore in a solid membrane, and then measure the optical signals when the particle encounters a beam of light in the channel. By correlating the strength of the current decrease as a particle moves through the pore, the intensity of the optical signal, and the time of each measurement, the researchers are able to discriminate among particles with different sizes and optical properties and to determine the flow speed of particles through the channel.
|
|
The chip can also be used to differentiate particles of similar size but different composition. In one experiment, the researchers combined influenza viruses with nanobeads of a similar diameter and placed the mixture above the nanopore. The virus was labeled with a red fluorescent tag and the beads were labeled with a blue tag. The researchers correlated the electrical signal with the fluorescent wavelength and the time of each measurement. They found that the blue nanobeads traveled faster through the channel than red influenza virus, perhaps because of a difference in surface charge or mass. Besides identifying pathogens in a mixture, the researchers can also count the number of virus particles.
|
|
"This could be used as an analytical device to do reliable counts of virus particles in a sample," Schmidt said.
|
|
Currently, Schmidt's group is working on methods to add optical trapping to the device. This would allow a molecule in the channel to be held in one place, investigated, and released, with the potential to analyze hundreds of molecules in an hour. "Having this all on one chip would make single-molecule measurements much easier and more convenient," Schmidt said.
|

