| Posted: Nov 11, 2014 |
Imaging with added sparkle
|
|
(Nanowerk News) Researchers from Cardiff University's Schools of Biosciences and Physics have unveiled a new method for viewing tiny diamonds inside living human cells. The pioneering technology could help to ensure that drugs are reaching the right target cells, as it enables researchers to see where a drug is reacting inside the body.
|
|
Nanodiamonds are small particles (a thousand times smaller than human hair) with low toxicity that can transport drugs inside cells. They show huge promise as an alternative to the organic fluorophores usually used by scientists to visualise processes inside cells and tissues.
|
|
A major limitation of organic fluorophores is that they degrade over time under light illumination. This makes it difficult to use them for accurate measurements of cellular processes. They can also become toxic or even kill cells. Nanodiamonds are one of the best inorganic material alternatives because of their compatibility with human cells, and due to their stable structural and chemical properties.
|
|
Previous attempts to visualise nanodiamonds have proved difficult as the diamond is transparent to visible light. Locating the nanodiamonds under a microscope had relied on tiny defects in the crystals that fluoresce under light illumination, but this can be unstable and difficult to read.
|
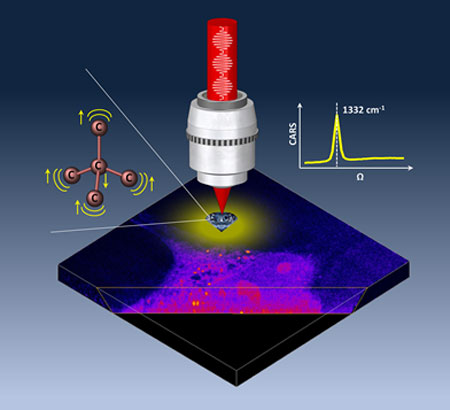 |
| Sketch of the vibrational resonances of diamond (inset left) and a CARS spectrum of a nanodiamond (right). The 3D image is acquired on a HeLa cell as an overlay of the CARS signal at the nanodiamond resonance (1332/cm) and the CARS signal from intracellular structures (2900/cm) where the relative intensities have been adjusted to enhance the cellular contrast for visualisation purposes. (Image: Cardiff University)
|
|
In their latest paper ("Coherent anti-Stokes Raman scattering microscopy of single nanodiamonds"), the researchers showed that non-fluorescing nanodiamonds (diamonds without defects) can be imaged far more stably via the interaction between illuminating light and the vibrating chemical bonds in the diamond's lattice structure, which results in scattered light of a different colour.
|
|
Two laser beams beating at a specific frequency are used to make chemical bonds vibrate in sync. One of these beams is then used to probe this vibration and generate light, called coherent anti-Stokes Raman scattering (CARS). By focusing these laser beams onto the nanodiamond a high-resolution CARS image is generated. Using an in-house built microscope, the researchers were able to measure the intensity of the CARS light on a series of single nanodiamonds of different sizes.
|
|
The nanodiamond size was accurately measured with electron microscopy and other quantitative optical contrast methods developed within the researcher's lab. In this way, they were able to quantify the relationship between the CARS light intensity and the nanoparticle size. Consequently, the calibrated CARS signal enabled the team to analyse the size and number of nanodiamonds that had been delivered into living cells with unprecedented accuracy.
|
|
Professor Paola Borri said: "This new imaging modality opens the exciting prospect of following complex cellular trafficking pathways quantitatively with important applications in drug delivery. The next step for us will be to push the technique to detect nanodiamonds of even smaller sizes than what we have shown so far and to demonstrate a specific application in drug delivery."
|

