| Posted: Dec 03, 2014 |
Nanoparticle network could bring fast-charging batteries
|
|
(Nanowerk News) A new electrode design for lithium-ion batteries has been shown to potentially reduce the charging time from hours to minutes by replacing the conventional graphite electrode with a network of tin-oxide nanoparticles.
|
|
Batteries have two electrodes, called an anode and a cathode. The anodes in most of today's lithium-ion batteries are made of graphite.
|
|
The theoretical maximum storage capacity of graphite is very limited, at 372 milliamp hours per gram, hindering significant advances in battery technology, said Vilas Pol, an associate professor of chemical engineering at Purdue University.
|
|
The researchers have performed experiments with a "porous interconnected" tin-oxide based anode, which has nearly twice the theoretical charging capacity of graphite. The researchers demonstrated that the experimental anode can be charged in 30 minutes and still have a capacity of 430 milliamp hours per gram (mAh g-1), which is greater than the theoretical maximum capacity for graphite when charged slowly over 10 hours.
|
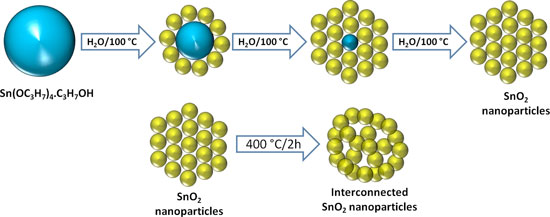 |
| This schematic diagram depicts the concept for a new electrode design for lithium-ion batteries that has been shown to potentially reduce the charging time from hours to minutes by replacing the conventional graphite electrode with a network of tin-oxide nanoparticles. (Purdue University image/Vinodkumar Etacheri)
|
|
The anode consists of an "ordered network" of interconnected tin oxide nanoparticles that would be practical for commercial manufacture because they are synthesized by adding the tin alkoxide precursor into boiling water followed by heat treatment, Pol said.
|
|
"We are not using any sophisticated chemistry here," Pol said. "This is very straightforward rapid 'cooking' of a metal-organic precursor in boiling water. The precursor compound is a solid tin alkoxide – a material analogous to cost-efficient and broadly available titanium alkoxides. It will certainly become fully affordable in the perspective of broad scale application mentioned by collaborators Vadim G. Kessler and Gulaim A. Seisenbaeva from the Swedish University of Agricultural Sciences."
|
|
Findings are detailed in a paper published in November in the journal Advanced Energy Materials ("Ordered Network of Interconnected SnO2 Nanoparticles for Excellent Lithium-Ion Storage").
|
|
When tin oxide nanoparticles are heated at 400 degrees Celsius they "self-assemble" into a network containing pores that allow the material to expand and contract, or breathe, during the charge-discharge battery cycle.
|
|
"These spaces are very important for this architecture," said Purdue postdoctoral research associate Vinodkumar Etacheri. "Without the proper pore size, and interconnection between individual tin oxide nanoparticles, the battery fails."
|
|
The research paper was authored by Etacheri; Swedish University of Agricultural Sciences researchers Gulaim A. Seisenbaeva, Geoffrey Daniel and Vadim G. Kessler; James Caruthers, Purdue's Gerald and Sarah Skidmore Professor of Chemical Engineering; Jeàn-Marie Nedelec, a researcher from Clermont Université in France; and Pol.
|
|
Electron microscopy studies were performed at the Birck Nanotechnology Center in Purdue's Discovery Park. Future research will include work to test the battery's s ability to operate over many charge-discharge cycles in fully functioning batteries.
|

