| Posted: Aug 17, 2015 |
In catalysis, a single atom makes all the difference
(Nanowerk News) Fuel cell technology offers a highly efficient and environmentally safe method of electricity generation. However, many of these cells require expensive Platinum catalysts. Therefore, to reduce the costs of fuel cells, more efficient catalysts must be synthesized.
|
|
Metal clusters containing small numbers of atoms are an exciting class of material that may offer catalytic performance superior to other coarse and ultrafine particles. Highly stable metal particles with a set number of atoms, known as 'magic number' clusters, are relatively easily synthesized.
|
|
However, it is thought that less stable, 'non-magic-number' clusters may exhibit enhanced catalytic activity.
|
|
A single atom makes all the difference
|
|
Unfortunately, investigation of these non-magic-number clusters has been hampered by the lack of a synthesis method that has single-atom precision.
|
|
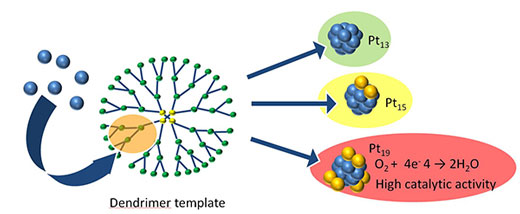
Kimihisa Yamamoto and co-workers at Tokyo Institute of Technology have now developed a new method, which allows fine control over the number of atoms in platinum clusters using branching molecules known as dendrimers ("Finding the most catalytically active platinum clusters with low-atomicity").
|
|
The effect of the number of atoms per cluster on catalytic performance was also investigated using the oxygen reduction reaction, an important reaction for energy conversion systems such as fuel cells.
|
|
Yamamoto's group previously accomplished synthesis of Pt12 and Pt13 clusters. Improving on this method, they can now prepare Pt12 through to Pt24 clusters with remarkable precision, by simply adjusting the amounts of dendrimer and metal precursor. This was demonstrated using two different dendrimers via stepwise and random routes.
|
 |
| Schematic of the stepwise dendrimer-templating strategy for synthesizing platinum clusters with a controlled number of atoms.
|
|
New synthesis method yields improved catalytic materials
|
|
The catalytic performance varied considerably with the number of atoms in the clusters, with Pt19 exhibiting the best performance. The experimental results and theoretical analysis reveal that larger clusters feature Pt13 cores with excess Pt atoms distributed at the edges. While symmetrical, magic-number Pt13 clusters have low catalytic activity, the addition of edge Pt atoms to this stable core produces clusters with unexpectedly high catalytic activity. The changes in activity are related to changes in the shape of the cluster that depend on the number of atoms.
|
|
Fueling catalytic performance
|
|
This work not only presents a synthesis route to prepare platinum clusters with single-atom control, but also sheds new light on structure-property relationships in metal clusters and highlights the great potential of non-magic-number clusters as high-performance catalysts. The new synthetic method presented and the catalytic performance measurements within the study are important steps in improving the performance of fuel cells.
|

