| Posted: Sep 02, 2015 |
Synthesizing nanodiamond-like carbon chains inside nanotubes
(Nanowerk News) The inner space of carbon nanotubes can act as a template for the synthesis of nanodiamond-like carbon chains. As a team of scientists from Japan, Germany, and the United States report in the journal Angewandte Chemie ("Template Synthesis of Linear-Chain Nanodiamonds Inside Carbon Nanotubes from Bridgehead-Halogenated Diamantane Precursors"), this templated polymerization approach paves the way for the design of novel one-dimensional nanomaterials.
|
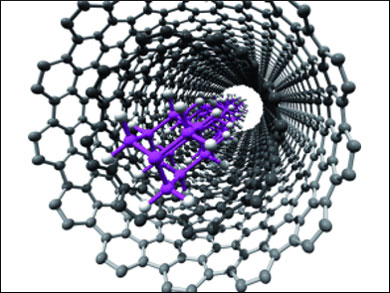 |
| Diamond-like Chains Inside Carbon Nanotubes
|
|
Nanosized materials such as nanowires offer unique properties that are completely distinct from those of the bulk materials. However, one-dimensional nanostructures are difficult to synthesize. In an international cooperation, Hisanori Shinohara from Nagoya University in Japan and his colleagues have developed a method that uses carbon nanotubes as a reaction vessel for the templated polymerization of linear-chain nanomaterials.
|
|
Template-Based Approach for 1D Structures
|
|
The idea was that during polymerization, the small precursor molecules would naturally adopt the one-dimensional structure of the tubes only if their inner diameter is small enough. Larger diameters would offer too much space so that the polymerization could terminate or become uncontrolled.
|
|
By using this method, Shinohara and his colleagues were able to synthesize a one-dimensional nanodiamond polymeric structure by a relatively simple annealing technique. They describe their approach: "The present template-based approach for the synthesis of linear-chain diamondoid polymers is entirely different from conventional chemical approaches."
|
|
Polymerization of Diamantane
|
|
The scientists used diamantane, a 10-carbon cage structure, as a precursor molecule and building block for polymerization. This molecule was brominated at either side so that, upon addition of iron nanoparticles, the bromine would be abstracted and a diradical formed.
|
|
In a normal chemical polymerization reaction, the formed radicals would abstract hydrogen for termination reactions, but: "To our great surprise, the radicals are persistent and recombined with each other inside the carbon nanotubes," the authors write. And: "Depending on the inner diameter of the carbon nanotubes, the inserted species can either be transformed into the linear-chain polymers or into amorphous carbon." As a result, the structures formed in the 1 nm-sized tubes were a polymeric chain of nanodiamondoids, which could be visualized impressively by electron microscopy.
|
|
To put it more colloquially, the formed carbon nanotubes filled with the nanodiamondoid polymer look like macaroni filled with spaghetti. In order to extract the inner polymer, a solution-phase sonication/extraction can be applied, the group reports. The big advantage of the new method is the simplicity and specificity of the formation of the one-dimensional nanostructured polymer chain. This technique will certainly attract the attention of materials scientists.
|

