| Posted: Sep 02, 2015 |
Cooperative carbon capture by a novel MOF material that mimics a plant enzyme
(Nanowerk News) Researchers discovered a novel metal-organic framework (MOF) material that exhibits an unprecedented cooperative mechanism for carbon dioxide (CO2) capture-and-release with only small shifts in temperature. This structure of the MOF, with CO2 adsorbed, closely resembles the RuBisCO enzyme found in plants, which captures CO2 from the atmosphere for conversion into nutrients ("Cooperative insertion of CO2 in diamine-appended metal-organic frameworks").
|
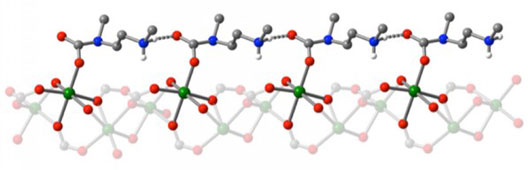 |
| Atomic structure of the adsorbed carbon dioxide (grey sphere bonded to two red spheres) inserted between the manganese (green sphere) and amine (blue sphere) groups within the novel metal-organic framework, forming a linear chain of ammonium carbamate (top). Some hydrogen atoms (white sphere) are omitted for clarity.
|
|
The discovery paves the way for designing more efficient materials that dramatically reduce overall energy cost of carbon capture. Such materials could be used for carbon capture from fossil-fuel-based power plants as well as from the atmosphere, mitigating the greenhouse effect.
|
|
A team of researchers at the Center for Gas Separations Relevant to Clean Energy Technologies, a DOE Energy Frontier Research Center led by the University of California, Berkeley, has discovered a cooperative mechanism for carbon dioxide (CO2) adsorption in porous MOF materials.
|
|
First, a CO2 molecule gets inserted between a metal ion and an amine group within the cylindrical pore of the MOF. Interestingly, the chemical environment of the MOF with the adsorbed CO2 is very similar to that of plant enzyme RuBisCO with a bound CO2. RuBisCO plays an essential role in biological carbon fixation by plants and conversion into nutrients. In the case of the newly synthesized diamine-appended MOFs, however, the inserted CO2 reorganizes the chemical environment at the adjacent metal ion site to be just right for the insertion of the next CO2. As more CO2 enters the pore, a cooperative domino effect ensues that leads to the formation of linear chains of ammonium carbamate along the cylindrical pore surfaces of the MOF.
|
|
Gas adsorption measurements show the high selectivity of the material for CO2 from the typical composition of flue gas from fossil-fuel-based power plants that contains nitrogen, water, and CO2. Furthermore, the material has large working capacities – the amount of CO2 adsorbed and desorbed for a given amount of material – that are enabled by only moderate temperature shifts for the adsorption and desorption processes.
|
|
Finally, the research points out that changing the strength of the metal-diamine bond through metal substitution allows for rational tuning of the adsorption and desorption properties.
|
|
The enhanced carbon capture efficiency of the new class of materials could allow for dramatic reductions in the overall energy cost of carbon capture in power plants or even from the atmosphere.
|

