| Posted: Oct 27, 2015 |
Promising technique improves hydrogen production of affordable alternative to platinum
(Nanowerk News) Scientists have demonstrated that microwaves can help create nanostructured molybdenum disulfide (MoS2) catalysts with an improved ability to produce hydrogen.
|
|
The microwave-assisted strategy accomplishes this by increasing the space, and therefore decreasing the interaction, between individual layers of MoS2 nanosheets. This exposes a larger fraction of reactive sites along the edges of these surfaces where hydrogen can be produced.
|
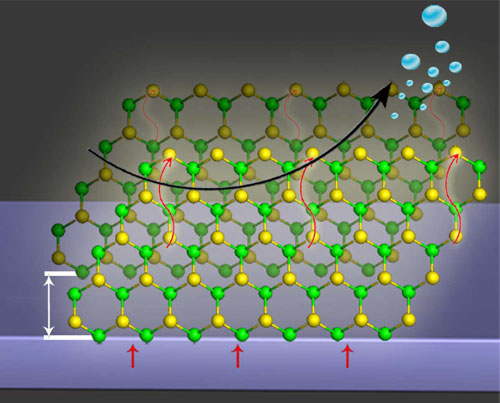 |
| A schematic representation of the edge-terminated MoS2 on glassy carbon electrode.
|
|
Atomistic first principles calculations show that the increase in spacing between the layers changes the electronic and chemical properties of these edge sites, making them more effective in producing hydrogen. The strategy was demonstrated by a small group of researchers at the Center for Nanoscale Materials (CNM), a U.S. Department of Energy (DOE) Office of Science User Facility based DOE’s Argonne National Laboratory.
|
|
"The microwave-assisted strategy could be a viable way to design advanced molybdenum disulfide catalysts for hydrogen production and hydrogen fuel cells," said Yugang Sun, a nanoscience scientist in Argonne's Nanoscience & Technology Division. "Microwave-synthesized nanostructured MoS2 exceeds the reactivity and stability levels of unmodified MoS2. Microwave-assisted synthesis is also a greener strategy when a compared to conventional heating methods."
|
|
Microwave energy is more efficient than conventional heating because it focuses its electromagnetic waves on only the material being treated and provides quicker, more even heating of a material's interior and exterior surfaces. Conventional or surface heating is slower than microwave heating and fails to achieve the desired result because it generates different temperatures in a material's interior compared to its surface area.
|
|
MoS2 is a common industrial catalyst that is used as a dry lubricant and in petroleum refining. It is one of a small handful of promising, Earth-abundant materials that could provide low-cost alternatives to platinum-based catalysts. Platinum is an extremely efficient catalyst for splitting water into hydrogen and oxygen, but its high-cost and scarcity limit its widespread use for hydrogen production and in hydrogen fuel cells.
|
|
This method will be extended to synthesize nanostructured MoS2 hybridized with other materials, which can strongly interact with MoS2 to influence its electronic structures and reactivity, to further improve the catalytic performance for producing hydrogen.
|
|
The research paper "Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production" was published in Nature Communications. Argonne's Min-Rui Goa, Maria K.Y. Chan and Yugang Sun are co-authors.
|
|
This research used several CNM capabilities including materials synthesis, electrocatalysis studies, their high-performance computing cluster Carbon, and characterization via X-ray diffraction, high-resolution transmission electron microscopy, Raman spectroscopy and Fourier transform infrared spectroscopy.
|

