| Posted: Nov 06, 2015 |
Light-absorbing polymers stand to attention
(Nanowerk News) Semiconducting polymers that absorb or emit light are used in solar cells and light-emitting diodes. But their optical properties can be difficult to predict, because they depend on how the polymer molecules are stacked together in thin films.
|
|
The flat, linear polymer molecules typically lie lengthways along a substrate, like a raft of logs. Making them stand vertically, as a ‘forest’ of polymer strands, is much more challenging but could dramatically affect the way they interact with light.
|
|
Now, Keisuke Tajima at the RIKEN Center for Emergent Matter Science and colleagues have developed a reliable technique for producing these vertically aligned polymer films, allowing them to study their behavior ("Optical Anisotropy and Strong H-Aggregation of Poly(3-Alkylthiophene) in a Surface Monolayer").
|
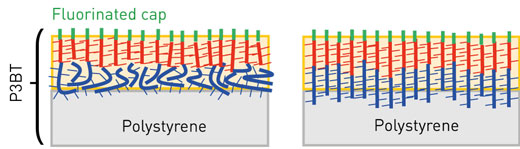 |
| Figure 1: A fluorinated cap causes strands of the polymer poly(3-butylthiophene) to pull away from a polystyrene film (left). On crystallizing, the strands line up vertically (right). (© Wiley)
|
|
The polymers possess clouds of -electrons that can interact both within their polymer strand and with neighboring strands. When these -conjugated polymers sit head-to-tail, interactions between their electrons tend to lengthen (redden) the wavelength of light that they absorb. In contrast, if side-by-side interactions dominate, the light will have a shorter (blue-shifted) wavelength. But the conformation of the chains can also have a dramatic effect. For example, poly(3-hexylthiophene) chains stretch out when they are crystallized in a thin film, causing their absorption wavelength to red-shift.
|
|
Tajima’s team created a polymer called P3BT-F17, composed of poly(3-butylthiophene) capped at one end with a chemical group loaded by fluorine atoms. When this polymer was mixed with polystyrene to form a thin film, the fluorinated groups separated from the polystyrene, pulling the poly(3-butylthiophene) sections into vertical alignment.
|
|
Optical measurements indicated that poly(3-butylthiophene) units next to the polystyrene base were laid flat and tended to absorb longer wavelengths, whereas those further away stood vertically and absorbed shorter wavelengths.
|
|
Heating the film rearranged the strands into a more ordered, crystalline structure, in which all the poly(3-butylthiophene) units were vertical (Fig. 1). This shifted their absorption to shorter wavelengths—probably due to strong interactions between the side-by-side chains. “We were very surprised,” says Tajima. “Such a large blue-shift after crystallization is the opposite of what we expected.”
|
|
The team then took two films of P3BT-F17 on polystyrene and sandwiched them together so that the fluorinated groups on the surface of each film were touching. This red-shifted the absorption by increasing the head-to-tail interactions of the poly(3-butylthiophene) units.
|
|
“We have shown that a vertical orientation of -conjugated polymers is possible in films and that they have unique electric and optical properties,” says Tajima. The researchers now hope to develop novel devices and discover new phenomenon based on these properties.
|

