| Posted: Feb 01, 2016 |
New type of nanowires, built with natural gas heating
(Nanowerk News) A team of Korean researchers, affiliated with UNIST has recently pioneered a new simple nanowire manufacturing technique that uses self-catalytic growth process assisted by thermal decomposition of natural gas. According to the research team, this method is simple, reproducible, size-controllable, and cost-effective in that lithium-ion batteries could also benefit from it.
|
|
In their approach, they discovered that germanium nanowires are grown by the reduction of germanium oxide particles and subsequent self-catalytic growth during the thermal decomposition of natural gas, and simultaneously, carbon sheath layers are uniformly coated on the nanowire surface.
|
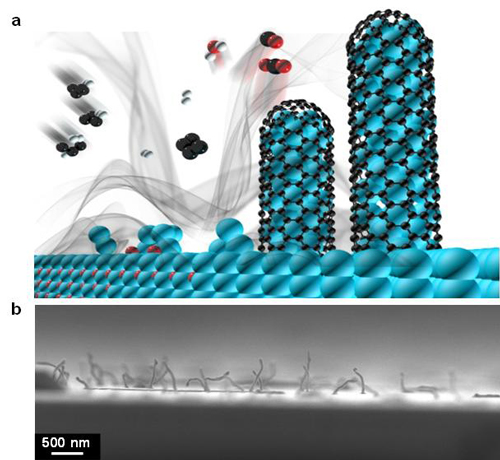 |
|
This study is a collaboration among scientists, including Prof. SooJin Park (School of Energy and Chemical Engineering) and Prof. Sang Kyu Kwak (School of Energy and Chemical Engineering), Dr. Sinho Choi (UNIST), Combined M.S./Ph.D. Student Dae Yeon Hwang (UNIST), and Researcher Jieun Kim (Korea Research Institute of Chemical Technology).
|
|
In a study, reported in the January 21, 2016 issue of Nano Letters ("Generalized Redox-Responsive Assembly of Carbon-Sheathed Metallic and Semiconducting Nanowire Heterostructures"), the team demonstrated a new redox-responsive assembly method to synthesize hierarchically structured carbon-sheathed germanium nanowires (c-GeNWs) on a large scale by the use of self-catalytic growth process assisted by thermally decomposed natural gas.
|
|
According to the team, this simple synthetic process not only enables them to synthesize hierachially assembled materials from inexpensive metal oxides at a larger scale, but also can likely be extended to other metal oxides as well. Moreover, the resulting hierarchically assembled nanowires (C-GeNWs) show enhanced chemical and thermal stability, as well as outstanding electrochemical properties.
|
|
The team states, "This strategy may open up an effective way to make other metallic/semiconducting nanomaterials via one-step synthetic reactions through an environmentally benign and cost-effective approach."
|

