| Posted: Feb 23, 2016 |
The inner light: Nanotechnology reveals density of tumors
(Nanowerk News) Tumor permeability can be a critical factor in how well a cancer therapy works. MSK researchers developed a method to measure this by creating living tumor spheroids and infiltrating them with carbon nanotubes that give off infrared light. The more light the tumor emits, the more permeable and potentially treatable it is.
|
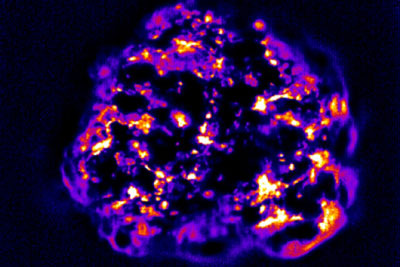 |
| Carbon nanotubes that have infiltrated a tumor give off light indicating the tumor’s permeability, which can be a critical factor in how well cancer drugs will work.
|
|
The above image, generated by the lab of Memorial Sloan Kettering pharmacologist Daniel Heller, shows a 3-D breast cancer tumor spheroid — a clump of human cells grown in a flask — that has been infiltrated by carbon nanotubes. These tiny, needle-like particles are about one nanometer thick.
|
|
The nanotubes give off light that reveals how permeable, or porous, the tumor is. This light, of infrared wavelength, is invisible to the eye but easily detectable by a special camera, even deep within tissues.
|
|
Tumor permeability can be a critical factor in how well a cancer therapy works. Different tumor types have different levels of permeability, depending on the density of the tumor’s extracellular matrix — a collection of molecules secreted by cells that provides structural and biochemical support. The nanotubes easily pass through a loose matrix but are foiled if it is too dense.
|
|
Some tumors are so dense that cancer drugs kill only the cells close to the surface, leaving the bulk of the tumor to thrive.
|
|
“The drug delivery aspect is often overlooked,” Dr. Heller says. “Sometimes a person is not responding to a drug because it’s not effective, or there is resistance. But sometimes it’s because the drug isn’t getting inside. It’s often hard to tell why it’s not working.”
|
|
The new approach of using nanotubes in living tumors exemplifies the immense potential of nanotechnology, an emerging field.
|
|
A wide range of nanomaterials are currently being investigated for their capacity to image cancer cells and to ferry drugs directly to tumors while avoiding healthy tissues. But researchers have been disappointed so far by these tools’ lack of clinical effectiveness in patients. One solution may be to figure out better ways to get nanomaterials — and drugs they may carry — deeper inside tumors.
|
|
Combining Two Technologies
|
|
Yosi Shamay, a research fellow in the Heller lab, created tumor spheroids by putting cancer cells in a plastic flask coated with a substance that prevents the cells from adhering to the sides so they instead stick to each other. The cells grow and clump together to form the spheroids, which resemble tumors found in living organisms.
|
|
Biophysicist Prakrit Jena developed a way to see the precise locations of infrared light-emitting carbon nanotubes within a three-dimensional volume. Dr. Heller worked with Dr. Shamay and Dr. Jena to use these carbon nanotubes in the tumor spheroids, creating a powerful new tool that adds another dimension — literally — to the study of cancer.
|
|
“Using conventional techniques, if you wanted to determine tumor porosity, you had to fix the tumor in paraffin, slice it, stain it, and study it under a microscope — it’s two dimensional,” Dr. Heller says. “Now we’ve devised a new method to get a live, real-time look at how well a substance is permeating a tumor.”
|
|
In a study reported recently in the journal Carbon ("Photoluminescent carbon nanotubes interrogate the permeability of multicellular tumor spheroids"), Dr. Heller’s lab used this technology to compare the permeability of a breast cancer spheroid with that of a liver cancer spheroid. The nanotubes demonstrated that the liver tumor was much denser, as infrared light was emitted only from its edges because the nanotubes could not penetrate deeply.
|
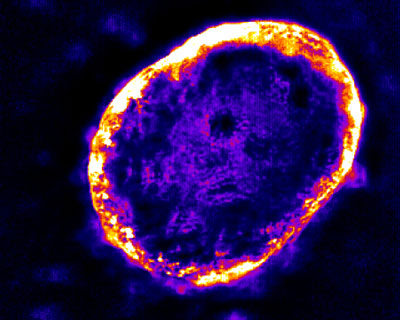 |
| Light confined to the edges indicates that the carbon nanotubes cannot penetrate far into the dense liver tumor.
|
|
When the researchers looked at the surface of both tumors under an electron microscope, they saw differences in extracellular matrix density that correlated with the extent of nanotube penetration — confirming that the amount of light emitted from inside the spheroids is a reliable indicator of the density of the matrix.
|
|
“We’re trying to make tumor spheroids for every cancer we can get our hands on to see how they behave,” Dr. Heller says.
|
|
Not only could this system shed light on tumor biology, it also could be used to screen for drugs that enhance tumor permeability. And the potential applications don’t end there.
|
|
“In addition to evaluating nanotubes and other nanomaterials, this is also a good method for studying penetration of other types of therapies, such as antibodies or viruses that target cancer,” Dr. Heller explains.
|
|
He says the technology might next be used to noninvasively peek inside a live tumor in a mouse.
|
|
“If we can take this into a mouse, and possibly one day into humans, it could be an important advance in figuring out better ways to get drugs inside a tumor,” he says. “This could be especially important for treating difficult cancers, which resist treatment even when detected early.”
|


