| Posted: Mar 02, 2016 |
Solving a nanotechnology riddle - what makes gold atoms stick together
(Nanowerk News) Gold is special, coveted as an investment, prized as jewellery and with a decorative history that dates back thousands of years. Ornate gilded surfaces have been found in ancient Egyptian tombs, where gold nanoparticles were used as paints.
|
|
Now researchers at UTS have solved the riddle of what makes gold special in today’s emerging field of nanotechnology.
|
|
Professor Jeffrey Reimers and Associate Professor Mike Ford, from the School of Mathematical and Physical Sciences, led a team that explained the chemical bonding process that occurs during the growth of gold nanoparticles.
|
|
Their research, published this week in the journal Proceedings of the National Academy of Sciences ("Gold surfaces and nanoparticles are protected by Au(0)–thiyl species and are destroyed when Au(I)–thiolates form"), paves the way for applications in biomedical imaging, drug delivery and electronics.
|
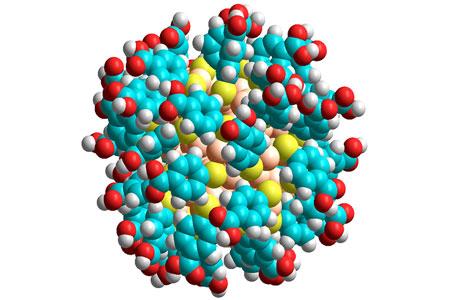 |
| A gold nanoparticle: gold atoms are coloured pale orange, sulphur yellow, oxygen red, carbon cyan and hydrogen white.
|
|
“What makes gold special – and, for that matter, what makes sulphur special – turned out to be the key in understanding how nanoparticles grow,” said Professor Reimers, who is a Fellow of the Australian Academy of Science and the 2016 winner of its David Craig Medal for Chemical Research.
|
|
“Gold is unique because it doesn’t rust, corrode or tarnish, meaning that it generally doesn’t react with the things around it. That’s why it’s known as a ‘noble metal’.
|
|
“The electrons in gold travel so fast they become heavy, an effect more important for gold than other atoms … so gold has the appearance of a metal, but with a strange colour and many more properties like those of non-metals such as sulphur.”
|
|
Developing nanoparticles into non-invasive and targeted treatments for diseases such as cancer is an ongoing challenge for scientists. The key lies in controlling the size and shape of gold nanoparticles, and making them behave in certain ways.
|
|
By identifying the significance of the “glue” that binds the surface of the gold nanoparticles to keep potentially destructive chemicals out of range, Professor Reimers and Associate Professor Ford, with collaborators from the Technical University of Denmark and the University of Sydney, have found the key that is critical to customising the properties of nanoparticles.
|
|
Gold and sulphur can react together to form strong covalent bonds (a chemical bond where electron pairs are shared between atoms) in compounds known as Au(I)-thiolates.
|
|
Professor Reimers said that for 30 years chemists have believed this to be the reason why sulphur glues stick to and protect gold nanoparticles.
|
|
“However, our research demonstrates that it is a force known as the van der Walls force - a type of attraction between molecules of quantum mechanical origin - that is responsible for binding sulphur to gold metal and nanoparticles.
|
|
“Until one properly and correctly understands the bonding, one cannot correctly describe the chemistry.”
|
|
Professor Reimers said the way was now open for people to design experiments that really tell how the nanoparticles grow.
|
|
“One can only imagine that given this knowledge, things can be made in the future that have never been dreamed about in the past.
|
|
“What we have now are better tools for understanding how to do these things, which will pave the way for researchers to invent new generations of gold nanotechnologies.”
|

