| Posted: May 13, 2016 |
Catalysis gets a metal-free upgrade
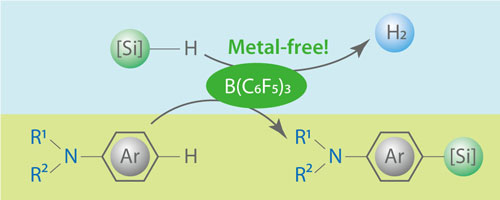
(Nanowerk News) Catalytic reactions between aromatic hydrocarbons and silicon compounds play critical roles in processes ranging from pharmaceutical production to coating surfaces with polymers. Now, an all-RIKEN team has discovered a surprisingly efficient way to eliminate the rare and toxic metals normally used in these transformations by employing a commercial boron catalyst ("Boron-catalyzed aromatic C–H silylation with hydrosilanes").
|
|
The reaction known as aromatic silylation, in which silicon-containing molecules called hydrosilanes are added to unsaturated carbon bonds on benzene-type rings, is normally too slow to be practical. To speed it up, chemists add tiny amounts of transition metals such as platinum and nickel. These catalysts act as intermediaries and simultaneously link to silicon and aromatic carbon reagents. This binding activates the reagents to connect into new organic–silicon compounds, while the metal catalyst separates and regenerates itself for further reactions.
|
 |
| A boron compound safely speeds up transformations between carbon and silicon. (Image: Zhaomin Hou, RIKEN Center for Sustainable Resource Science)
|
|
Zhaomin Hou and colleagues from the RIKEN Center for Sustainable Resource Science realized that a metal-free technique for catalyzing carbon–silicon bond formation could reap dividends by reducing the costs associated with recovering trace metals, which are detrimental to both human health and electronic device operation. Achieving this, however, required rethinking standard synthetic strategies.
|
|
“Most of our knowledge is based on metal-catalyzed reactions,” explains Hou. “But metal and non-metal catalysts usually work in different mechanisms, and it is not so straightforward to design new reactions.”
|
|
The team’s experience with a commercially available compound known as tris(pentafluorophenyl)borane, or BCF, proved valuable. The boron atom in BCF is electron deficient, making it useful to activate stubbornly stable bonds, such as those between silicon and hydrogen atoms. While Hou and colleagues had previously used BCF as a co-catalyst with rare earths, they suspected it warranted its own investigation.
|
|
Screening of BCF revealed it had a knack for adding hydrosilanes to aromatic carbon–hydrogen bonds (Fig. 1). Using no additives other than the boron catalyst, the researchers successfully linked a wider range of reagents, including bulky molecules and ones with reactive chlorine bonds, than is possible with metal catalysts. Furthermore, additions occurred at specific positions on the aromatic reagent frameworks—a boon for chemists seeking to maximize the purity of their reaction products.
|
|
“We have been using boron compounds as co-catalysts for years,” says Hou. “But to discover that a simple boron compound alone can efficiently catalyze the carbon–hydrogen silylation, with substrate scope far beyond those of metal catalysts, was really surprising and exciting.”
|
|
The researchers are investigating whether this environmentally benign protocol has further applications in C–H functionalization reactions.
|

