| Posted: May 19, 2016 |
It takes two to make an electrode go right
(Nanowerk News) With more capacity and fewer safety issues than their lithium counterparts, magnesium batteries are potentially a promising energy storage option, but the electrodes are difficult to produce and quickly fail. Scientists want something better.
|
|
Inspired by a two-metal electrode, made of tin and antimony, a team at DOE's Pacific Northwest National Laboratory (PNNL) delved into the atomic workings of this alloy (Advanced Materials, "Interface Promoted Reversible Mg Insertion in Nanostructured Tin-Antimony Alloys"). They saw the metals separate into antimony- and tin-rich regions. The tin regions worked well; the antimony-rich areas did not. However, the antimony regions were crucial: at the interface, or border, between the two regions, the antimony kept the tin structure from collapsing.
|
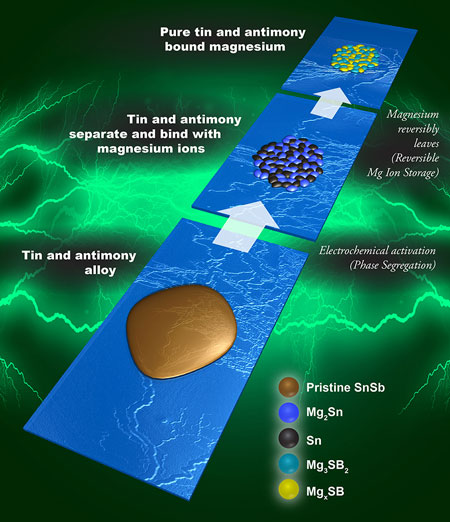 |
| In a tin and antimony alloy, a potential electrode for magnesium batteries, the metals separate when the battery is first charged. Both metals bind with magnesium ions, but only the tin regions release them. However, the antimony serves a vital role in stabilizing the overall structure. (Image: Cortland Johnson, PNNL)
|
|
This work has applications beyond batteries. "More generally, this research is developing new knowledge on how to synthesize materials with certain properties and certain structures," said Dr. Maria Sushko, computational physicist at PNNL. "The Office of Science at DOE provided the environment to do research in such broad areas."
|
|
Why It Matters
|
|
One of the concerns with electric cars is long, lonely stretches of highway. Drivers don't want to be stranded between charging stations. This concern can factor into their decision to buy lower emission vehicles. The results of this study add to the scientific understanding of how to create materials for better magnesium batteries and other energy storage devices.
|
|
"This research offers important information on how to start with an alloy and create a phase-separated material with a completely different structure that is useful for energy technologies," said Dr. Yuyan Shao, a chemist on the team.
|
|
Methods
|
|
In batteries, certain ions must move in and out of the electrode with each use. If an electrode fails to welcome or release the ions, the battery fades and fails. In 2015, Dr. Jun Liu and Dr. Nigel Browning led a study (Nano Letters, "Realizing the Full Potential of Insertion Anodes for Mg-Ion Batteries Through the Nanostructuring of Sn") at PNNL that showed how tin and antimony segregate into different regions in an electrode. The team at PNNL took this work further to uncover the processes taking place at the interfaces between these regions and to find the origin of electrode stability.
|
|
The team used experiments and theory to determine how the crystal structure evolves in the antimony- and tin-rich regions during charging and discharging, as would be seen in a battery. When a magnesium ion slides into the tin region and later leaves, it does not cause the collapse seen in pure tin. When the ion moves into the antimony-rich region, it becomes locked into the structure. This makes the antimony portion a poor electrode, but it is the antimony region that keeps the whole electrode stable.
|
|
The alloy electrode delivers a high reversible capacity and remains stable over hundreds of rounds of discharging and charging. Moreover, the team demonstrated that the alloy electrodes worked well with other magnesium battery components, specifically the simple salt electrolytes. This makes the alloy electrodes more attractive and opens a new avenue to explore magnesium batteries.
|
|
This year-long research project was possible because of the team's diverse expertise in chemistry, materials sciences, engineering, and physics. "The Office of Science provided opportunities for us to work together," said Shao. "They also provided state-of-the-art capabilities at the EMSL user facility."
|
|
What's Next?
|
|
The PNNL team is planning to put the alloy into a full battery system and see how it performs. On a broader scale, this work will help others understand interfaces in fuel cells, nuclear reactors, and other energy storage and production systems.
|

