| Posted: Jul 12, 2016 |
Silk-based tissue chip provides promise for drug testing and implantable devices
(Nanowerk News) Researchers have created a new type of tissue chip that can better represent human tissues compared with current chips, and can be more widely used for drug testing. By engineering the chips as a silk gel, the researchers circumvented many of the problems with existing devices. The new chip also has the potential to someday be an implantable treatment itself.
|
|
Tissue chips are collections of cells that mimic both the anatomy and physiology of a tissue or organ, making it possible to test treatments in the lab more accurately than using cells grown in a single layer in a dish. To engineer a tissue outside the body, the cells need a three-dimensional structure on which to grow. Such scaffolds are often made of polydimethylsiloxane (PDMS), a silicon-based polymer, and contain microfluidic chambers, representing blood vessels or respiratory tracts, running through them.
|
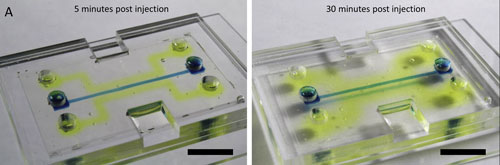 |
| The enzyme alkaline phosphatase is injected into the silk chip and remains biologically active (yellow) as it diffuses into the silk hydrogel after five minutes (left) and 30 minutes (right). (Source: Elsevier) (click on image to enlarge)
|
|
These microfluidic systems have various advantages. Some systems are great for developing and testing treatments in the lab; some allow living cells to be embedded within them, while others can replicate a variety of tissue types (bone and bone marrow, say). Other systems have qualities that may allow them to be implanted in the body as part of the treatment itself; one such quality is the ability to eventually degrade away when no longer needed. But, none of the current biomaterials can do all of the above. PDMS is particularly problematic because it is non-degradable, and it sucks up lipids, such as fat molecules or steroid hormones. Many potential medications are lipid based, so PDMS absorbs them before their effects can be measured, making it difficult to test drugs. Additionally, an implant made of PDMS would absorb the body’s lipids, and since lipids are vital to the body’s function, a PDMS microchip can’t be implanted in humans.
|
|
To create a system that addresses all of these needs, researchers turned to silk, a naturally derived protein with unique properties that have several benefits: provide different levels of stiffness to match the target tissue; afford long-term stability in a variety of conditions yet still fully degrade over time; and offer transparency so researchers can observe biological processes like enzymatic activity.
|
|
“We know that silk is biocompatible so you can use it even inside the body, and it can be programmed to dissolve over time safely,” said Rosemarie Hunziker, Ph.D., program director for Tissue Engineering at NIBIB. “So this might even be an improved design that enables us to build little micro-tissues and make them implantable.” The silk-based system was described online on March 31, 2016 in the journal Biomaterials ("Bio-functionalized silk hydrogel microfluidic systems").
|
|
Researchers from the Department of Biomedical Engineering at Tufts University in Medford, Massachusetts developed the microfluidic device by mixing silk into a gel solution and casting it into a mold. This created a rectangular block of silk hydrogel with a three-dimensional network of channels running through it. Mechanical valves were also added to control flow through the channels; the flow could be switched on or off based on the air pressure within one of the chambers.
|
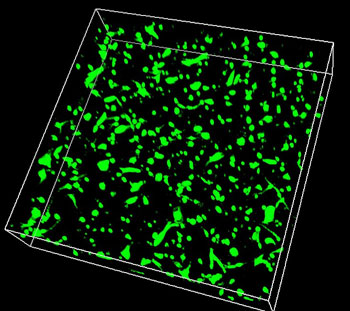 |
| 3D image of skin cells successfully growing in the biocompatible silk hydrogel. (Image: Siwei Zhao)
|
|
In living tissues and organs, interactions with other cells, proteins, and enzymes occur both within the tissue and on the surface of the channels. Modeling this involves embedding living cells and active enzymes within the gel while it’s made. However, the harsh conditions required to create PDMS kill and deactivate cells and enzymes. Because a silk hydrogel can be made at ambient temperatures and under relatively gentle conditions, it can include cells and enzymes within the gel and thus better replicate living tissue.
|
|
Silk gels were also able to withstand a variety of environments (such as changes to the surrounding fluid’s pH or salinity) without altering their size or shape. On the other hand, the stiffness of the gel could be manipulated to match the properties of the target tissue (harder for cartilage, but soft for skin or brain, for example). The gels were also clear, allowing for easier analysis.
|
|
While testing potential drugs is the likely first application of the silk system, David Kaplan, Ph.D., Stern Family Professor of Engineering at Tufts University and senior author of the paper, is also excited about the possibility of someday growing tissues on chips that can be put into the body. “Silk takes you to the next level because it can be implanted and fully resorbed in vivo,” said Kaplan.
|
|
And for researchers looking for a system that can be tailored to a specific need—whether it’s mechanical pumps, cell signaling, or imaging of cellular processes within the chip—this is it, said Kaplan. “Silk is pretty unique in the ability to integrate everything into one material system,” he said. “Now we can optimize systems in vitro (in cell culture) and directly translate that in vivo (within an animal) to look at tissue regeneration. I don’t know of any other system with the versatility that can do all that.”
|
|
Kaplan is known for using silk to solve biomedical engineering problems; he’s used it to make models of brain tissue and bone marrow, as part of surgical implants to heal broken bones, and as a method for keeping antibodies and vaccines stable at room temperature. “It’s pretty rare when we hit a roadblock that we can’t overcome with silk as the base material,” said Kaplan. “It’s a fairly universal material. I’m hopeful we’ve moved it out of the textile world and into the biomaterials and medical world.”
|
|
Indeed, compared to other polymers being tested, silk is well studied. “We already know a lot about how it reacts inside the body,” said Hunziker. In terms of developing silk-based tissue implants, “It’s like starting a relay race on the last lap instead of from the beginning.”
|


